Joint modeling of predictors of adverse drug reactions and viral load changes over time among adult patients on ART at Nigist Eleni Mohammed Memorial comprehensive specialized hospital, central Ethiopia
- PMID: 40652201
- PMCID: PMC12256003
- DOI: 10.1186/s12879-025-11294-7
Joint modeling of predictors of adverse drug reactions and viral load changes over time among adult patients on ART at Nigist Eleni Mohammed Memorial comprehensive specialized hospital, central Ethiopia
Erratum in
-
Correction: Joint modeling of predictors of adverse drug reactions and viral load changes over time among adult patients on ART at Nigist Eleni Mohammed Memorial comprehensive specialized hospital, central Ethiopia.BMC Infect Dis. 2025 Aug 21;25(1):1054. doi: 10.1186/s12879-025-11478-1. BMC Infect Dis. 2025. PMID: 40841891 Free PMC article. No abstract available.
Abstract
Background: Adverse drug reactions (ADRs) represent a significant global public health concern, contributing substantially to patient morbidity and mortality. While viral load dynamics are known to influence ADRs incidence, this relationship has been insufficiently explored in clinical research. Current evidence is limited by methodological constraints, as most prior studies have analyzed these factors separately rather than employing integrated approaches that account for the endogenous nature of time-varying biomarkers like viral load. Joint modelling techniques offer a robust solution by simultaneously analyzing longitudinal and time-to-event data, providing more efficient and precise estimates of this critical association. This study aimed to determine the incidence and predictors of ADRs and its association with changes in viral load among adult patients on antiretroviral therapy (ART) at Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital (NEMMCSH) of central Ethiopia.
Methods: A retrospective follow-up study at NEMMCSH from March 1, 2018 to April 30, 2022 among 376 randomly selected age above 15 years patients who initiated first-line ART and had two or more viral load measurements during follow-up. Data were extracted from medical records and analyzed using joint modeling techniques combining Cox proportional hazards regression with linear mixed effects models. Results are presented as adjusted hazard ratios (aHR) with 95% confidence intervals. Model adequacy was assessed through residual diagnostics, and statistical significance was determined at α = 0.05.
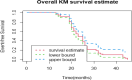
Result: A total of 376 adult patients on ART were followed for a combined 45,752 person-months of observation (median follow-up = 23 months; range: 1-50 months), accounting for variability in enrollment dates and censoring events. Overall, 93 patients (26.70%) developed ADRs with an incidence rate of 14.2 per 1000 person-months observation (95% CI: 13.20-15.40). The ART regimen (AZT/3TC/NVP) (AHR = 2.15: 95% CI: 1.01-4.57), TB co-infection (AHR = 0.79: 95% CI: 0.68-0.87) and ART duration (AHR = 0.96: 95% CI: 0.95-0.97) were significant predictors of ADRs. The time-dependent true value of the viral load log was significantly associated with the risk of adverse drug reaction (α = 1.67: AHR = 5.31: 95% CI: 1.64-7.23).
Conclusion: ADRs among HIV patients continue to be a public health concern. Viral load changes were significant associations with the risk of adverse reactions. To reduce ART adverse reactions, we recommend health professionals closely monitor and follow those patients on ART regimens (AZT/3TC/NVP).
Keywords: Adverse drug reaction; Anti-retroviral therapy; HIV/AIDS; Joint model; Viral load.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Research Ethics Review Committee (RERC) board of the University of Gondar, College of Medicine and Health Sciences (Reference Number: UOG/00231/14). The committee waived the requirement for individual informed consent due to the retrospective nature of the study, which utilized anonymized secondary data extracted from medical records. No personal identifiers were collected, and all data were kept confidential and used solely for research purposes. The study adhered to the principles of the Declaration of Helsinki and complied with national ethical guidelines for research involving human subjects. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Global HIV. & AIDS statistics — Fact sheet https://https://www.unaids.org/en/resources/fact-sheet 2023.
-
- UNAIDS. Global HIV/AIDS statistics– 2020 Fact sheet Ι UNAIDS https://www.unaids.org/en/resources/fact-sheet [Cited 23/01/2021]. 2020.
-
- Ethiopian Federal Ministry of Health. National guidelines for comprehensive HIV prevention, care and treatment. 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

