Association between hemoglobin glycation index and mortality in critically ill patients: a retrospective cohort study
- PMID: 40652238
- PMCID: PMC12254999
- DOI: 10.1186/s41043-025-01008-9
Association between hemoglobin glycation index and mortality in critically ill patients: a retrospective cohort study
Abstract
Background: Glycemic variability is increasingly recognized as a critical factor influencing outcomes in intensive care, yet its prognostic role remains unclear. The Hemoglobin Glycation Index (HGI), which reflects individual glycemic variation, has not been thoroughly studied in critically ill populations.
Aim: To evaluate the association between HGI and all-cause mortality in critically ill patients using data from a large intensive care unit (ICU) cohort.
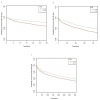
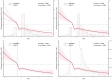
Methods: We conducted a retrospective cohort study using the MIMIC-IV database. The primary outcomes were 30-, 90-, and 365-day all-cause mortality; in-hospital mortality was secondary. Kaplan-Meier analysis, Cox regression, and restricted cubic spline (RCS) modeling were used to assess mortality risk across HGI levels. Propensity score matching (PSM) and subgroup analyses were performed to ensure robustness.
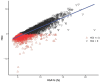
Results: Among 9,695 patients, those with low HGI (< - 0.40) had significantly higher mortality (P < 0.001). RCS analysis showed a nonlinear association between HGI and 30-day mortality. Higher HGI values were independently associated with reduced risk of death at all time points, with hazard ratios ranging from 0.43 to 0.76 (P < 0.001). These associations persisted after multivariable adjustment and PSM. Subgroup analyses showed consistent results across patient characteristics.
Conclusions: Lower HGI values are associated with increased short- and long-term mortality in critically ill patients. HGI may serve as a valuable prognostic biomarker for risk stratification in ICU settings.
Keywords: Hemoglobin glycation index; Intensive care unit; Mortality; Restricted cubic spline; Retrospective cohort study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Ethical approval for the MIMIC-IV database was granted by the institutional review boards at Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology. Given the absence of protected health information in the database, the institutional review board approval included a waiver of the informed consent requirement.
Figures
References
-
- Serpa Neto A, Bailey M, Seller D, Agli A, Bellomo R, Brickell K, Broadley T, Buhr H, Gabbe BJ, Gould DW, et al. Impact of High-Dose early mobilization on outcomes for patients with diabetes: A secondary analysis of the TEAM trial. Am J Respir Crit Care Med. 2024;210(6):779–87. - PubMed
-
- Kamei J, Yamamoto S. Complicated urinary tract infections with diabetes mellitus. J Infect Chemotherapy: Official J Japan Soc Chemother. 2021;27(8):1131–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous