Deficiency of calretinin in prefrontal cortex causes behavioral deficits relevant to autism spectrum disorder in mice
- PMID: 40652246
- PMCID: PMC12255998
- DOI: 10.1186/s13041-025-01233-7
Deficiency of calretinin in prefrontal cortex causes behavioral deficits relevant to autism spectrum disorder in mice
Abstract
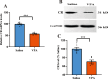
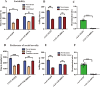
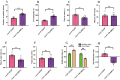
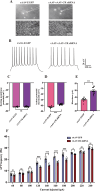
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by core symptoms including deficits in social interaction, repetitive and stereotyped behaviors, along with higher levels of anxiety and cognitive impairments. Previous studies demonstrate pronounced reduced density of calretinin (CR)-expressing GABAergic interneurons in both ASD patients and animal models. The object of the current study was to determine the role of CR in ASD-relevant behavioral aberrations. Herein, the mRNA and protein levels of CR in the prefrontal cortex (PFC) of mouse model of ASD based on prenatal exposure to valproic acid (VPA) were determined by qRT-PCR and Western blot analysis, respectively. Moreover, the behavioral abnormalities in naive mice with CR deficiency mediated by recombinant adeno-associated virus (rAAV) were evaluated in a comprehensive testing battery including social interaction, marble burying, self-grooming, open-field, elevated plus maze and novel object recognition tests. Furthermore, the action potential changes caused by CR deficiency were examined in neurons within the PFC in naive mouse. The results show that the mRNA and protein levels of PFC CR of VPA-induced mouse ASD model were reduced. Concomitantly, mice with CR knockdown displayed ASD-like behavioral aberrations, such as social impairments, elevated stereotypes, anxiety and memory defects. Intriguingly, patch-clamp recordings revealed that CR knockdown provoked decreased neuronal excitability by increasing action potential discharge frequencies together with decreased action potential threshold and rheobase. Our findings support a notion that CR knockdown might contribute to ASD-like phenotypes, with the pathogenesis most likely stemming from increased neuronal excitability.
Keywords: Action potential; Autism spectrum disorder; Calretinin; Mouse model; Prefrontal cortex.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experimental procedures were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Zhengzhou University. Written informed consent was obtained from the owners for the participation of their animals. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
GABAB1 receptor knockdown in prefrontal cortex induces behavioral aberrations associated with autism spectrum disorder in mice.Brain Res Bull. 2023 Oct 1;202:110755. doi: 10.1016/j.brainresbull.2023.110755. Epub 2023 Sep 7. Brain Res Bull. 2023. PMID: 37678443
-
Arecoline alleviates autism spectrum disorder-like behaviors and cognition disorders in a valproic acid mouse model by activating the AMPK/CREB/BDNF signaling pathway.Brain Res Bull. 2025 Sep;229:111431. doi: 10.1016/j.brainresbull.2025.111431. Epub 2025 Jun 11. Brain Res Bull. 2025. PMID: 40513625
-
Hippocampal Morphological Alterations and Oxidative Stress in Autism Spectrum Disorder Model Induced by Prenatal Exposure to Valproic Acid in Male and Female Mice.Hippocampus. 2025 Jul;35(4):e70024. doi: 10.1002/hipo.70024. Hippocampus. 2025. PMID: 40626515
-
Methylphenidate for children and adolescents with autism spectrum disorder.Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011144. doi: 10.1002/14651858.CD011144.pub2. Cochrane Database Syst Rev. 2017. PMID: 29159857 Free PMC article.
-
Behavioural and cognitive behavioural therapy for obsessive compulsive disorder (OCD) in individuals with autism spectrum disorder (ASD).Cochrane Database Syst Rev. 2021 Sep 3;9(9):CD013173. doi: 10.1002/14651858.CD013173.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693989 Free PMC article.
References
-
- Klein ME, Bangerter A, Halter RJ, Cooper K, Aguilar Z, Canuso CM, Drevets WC, Schmidt ME, Pandina G. Efficacy and safety of JNJ-42165279, a fatty acid amide hydrolase inhibitor, in adolescents and adults with autism spectrum disorder: a randomized, phase 2, placebo-controlled study. Neuropsychopharmacology. 2024;50(2):480–7. - PMC - PubMed
-
- Kurahashi H, Kunisawa K, Tanaka KF, Kubota H, Hasegawa M, Miyachi M, Moriya Y, Hasegawa Y, Nagai T, Saito K, et al. Autism spectrum disorder-like behaviors induced by hyper-glutamatergic NMDA receptor signaling through hypo-serotonergic 5-HT1A receptor signaling in the prefrontal cortex in mice exposed to prenatal valproic acid. Neuropsychopharmacology. 2024;50(5):739–50. - PMC - PubMed
-
- Han YMY, Chan MMY, Shea CKS, Lai OLH, Krishnamurthy K, Cheung M-C, Chan AS. Neurophysiological and behavioral effects of multisession prefrontal tDCS and concurrent cognitive remediation training in patients with autism spectrum disorder (ASD): a double-blind, randomized controlled fNIRS study. Brain Stimul. 2022;15(2):414–25. - PubMed
-
- Gillespie B, Panthi S, Sundram S, Hill RA. The impact of maternal immune activation on GABAergic interneuron development: a systematic review of rodent studies and their translational implications. Neurosci Biobehav Rev. 2024;156:105488. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

