Obesity induces phenotypic switching of gastric smooth muscle cells through the activation of the PPARD/PDK4/ANGPTL4 pathway
- PMID: 40652248
- PMCID: PMC12254972
- DOI: 10.1186/s12929-025-01163-5
Obesity induces phenotypic switching of gastric smooth muscle cells through the activation of the PPARD/PDK4/ANGPTL4 pathway
Abstract
Background: Clinical research has identified stomach dysmotility as a common feature of obesity. However, the specific mechanisms driving gastric emptying dysfunction in patients with obesity remain largely unknown. In this study, we investigated potential mechanisms by focusing on the homeostasis of gastric smooth muscle.
Methods: An obese mouse model was established using a high-fat diet (HFD). Immunofluorescence analysis and Western blotting were employed to assess smooth muscle status using stage-specific markers. An in vitro culture model of differentiated human gastric smooth muscle cells (SMCs) was treated with lipids, siRNA-peptide-based nanoparticles and pharmaceutical compounds. Global lipidomic and RNA sequencing analyses were performed. The findings were evaluated in patients with obesity, using gastric samples from individuals who underwent sleeve gastrectomy, to evaluate their clinical relevance.
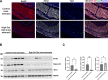
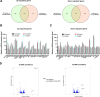
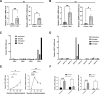
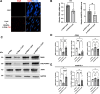
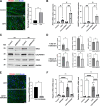
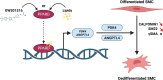
Results: The smooth muscle layers in gastric tissue from both mice fed on a HFD as well as patients with obesity exhibited altered differentiation status. Treatment of differentiated human gastric SMCs with lipids phenocopies these alterations and is associated with increased expression of PDK4 and ANGPTL4. Inhibition of PDK4 or ANGPTL4 upregulation prevents these lipid-induced modifications. PPARD activation stimulates PDK4 and ANGPTL4 upregulation, leading to SMC dedifferentiation. Notably, PDK4 and ANGPTL4 levels correlate with immaturity and alteration of gastric smooth muscle in patients with obesity.
Conclusions: Obesity triggers a phenotypic change in gastric SMCs, driven by the activation of the PPARD/PDK4/ANGPTL4 pathway. These mechanistic insights offer potential biomarkers for diagnosing stomach dysmotility in patients with obesity.
Keywords: Immaturity; Obesity; PDK4/ANGPTL4/PPARD pathway; Smooth muscle; Stomach.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study involves human participants. The biological collection was registered with the French Ministry (numbers DC 2015–2473 and AC 2016–2760). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Afshin A, Reitsma MB, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries. N Engl J Med. 2017;377:1496–7. - PubMed
-
- Delgado-Aros S, Locke GR, Camilleri M, Talley NJ, Fett S, Zinsmeister AR, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

