Prognostic value of LncRNA PSMA3-AS1 in prostate cancer and its potential regulatory mechanism
- PMID: 40652273
- PMCID: PMC12255973
- DOI: 10.1186/s41065-025-00485-6
Prognostic value of LncRNA PSMA3-AS1 in prostate cancer and its potential regulatory mechanism
Abstract
Objective: Prostate adenocarcinoma (PRAD) is asymptomatic in the early stages and most patients are diagnosed at an advanced stage, which leads to a poor prognosis. Therefore, an effective prognostic marker is required to improve PRAD prognosis.
Methods: A total of 128 patients with PRAD were included in the study. PSMA3-AS1 and miR-29a-3p expression in tissues was detected using RT-qPCR. CCK-8 and Transwell assays were then used to evaluate the proliferative, migratory, and invasive capacities of prostate cancer cell lines. A DLR assay confirmed the binding relationship between PSMA3-AS1 and miR-29a-3p. The five-year prognosis of PRAD patients was analyzed using a Kaplan-Meier plotter curve.
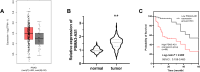
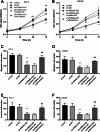
Results: PSMA3-AS1 was highly expressed in PRAD tissues, and patients with high expression had poor 5-year survival. In contrast, miR-29a-3p was poorly expressed in PRAD tissues. PSMA3-AS1 bound to miR-29a-3p in a targeted manner and the levels showed a negative correlation. Knocking down PSMA3-AS1 could increase the level of miR-29a-3p and slow the proliferation of PRAD cell lines, as well as inhibiting their migration and invasion ability.
Conclusion: A high level of PSMA3-AS1 was strongly linked to a poor prognosis for patients and is expected to serve as a prognostic marker for PRAD. Furthermore, PSMA3-AS1 knockdown increased the level of miR-29a-3p and reduced the physiological activity of cancer cells. Therefore, regulating the expression of the PSMA3-AS1/miR-29a-3p axis could influence PRAD development.
Keywords: PSMA3-AS1; Prognostic; Prostate adenocarcinoma; miR-29a-3p.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shanxi Province Cancer Hospital before the study began. The written informed consent has been obtained from the participants involved. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures
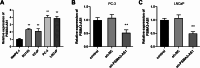



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

