Performance and usability evaluation of three LDH-based malaria rapid diagnostic tests in Kédougou, Senegal
- PMID: 40652282
- PMCID: PMC12255971
- DOI: 10.1186/s13071-025-06914-9
Performance and usability evaluation of three LDH-based malaria rapid diagnostic tests in Kédougou, Senegal
Abstract
Background: The emergence of Plasmodium falciparum (Pf) parasites with deleted histidine-rich protein 2 and 3 (hrp2/hrp3) genes threatens the performance of HRP2-based malaria rapid diagnostic tests (RDTs). RDTs targeting Pf lactate dehydrogenase (LDH) may address current product limitations and improve case management. The objective of this study was to evaluate the performance and usability of three LDH-based RDTs in febrile patients.
Methods: A cross-sectional diagnostic accuracy study was conducted in Kédougou, Senegal. Capillary blood was tested using the SD BIOLINE Ag Pf RDT (Abbott Diagnostics Korea Inc., Republic of Korea) and three LDH-based RDTs (BIOCREDIT Malaria Ag Pf [pLDH], BIOCREDIT Pf [pLDH/HRPII] and BIOCREDIT Pf/Pv [pLDH/pLDH]; Rapigen Inc., Republic of Korea). Venous blood was collected and used to repeat the BIOCREDIT RDTs and conduct microscopy. Frozen venous specimens were tested using a reference PCR assay. A quantitative multiplex malaria antigen assay measured antigen concentration. RDT performance was determined and analyzed as a function of antigen concentration distribution. Usability of the Pf-only BIOCREDIT tests was evaluated using a questionnaire.
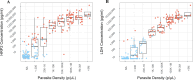
Results: Of the 220 participants, 154 (70%) were Pf-positive by PCR. The Pf (pLDH/HRPII) test demonstrated the highest sensitivity at 78% (95% confidence interval [CI] 70.9-84.5%); specificity was 89% (95% CI 79.4-95.6%). All RDTs performed better than microscopy (53% sensitivity). Although the HRP2 line on the Pf (pLDH/HRPII) test was more sensitive than the SD BIOLINE HRP2-based test (71%, 95% CI 63.6-78.4%), the sensitivities of the PfLDH lines alone on all three BIOCREDIT tests (61-64%) were lower than that of the SD BIOLINE HRP2 test. RDTs performed better when compared to antigen concentration over PCR results. Improved sensitivity of the Pf (pLDH/HRPII) test was driven by the HRP2 line. Line intensity correlated with antigen concentration. Predicted sensitivity using the analytical limit of detection (LOD) was comparable to the observed sensitivity. RDTs demonstrated acceptable usability.
Conclusions: Both HRP2 and LDH contributed to the sensitivity of the best-performing Pf-RDT. In populations such as this with low rates of hrp2/hrp3 deletions, the PfLDH line alone cannot compensate for the performance of the HRP2 line, even with the improved PfLDH LOD of the BIOCREDIT tests. RDT analytical LODs can be used to predict performance in populations with known antigen concentrations.
Keywords: Diagnosis; Histidine rich protein 2; Lactate dehydrogenase; Malaria; Rapid diagnostic test.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was reviewed and approved by the Institutional Review Boards (IRBs) of Comité National d'Ethique pour la Recherche en Santé (CNERS) [00000126/MSAS/CNERS/SP], Sénégal, and the WIRB-Copernicus Group (WCG) [1313427]. Written informed consent was obtained from all study participants. For minors, consent was provided by legal guardians, and children over the age of 7 years also provided assent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Update of
-
Performance and usability evaluation of three LDH-based malaria rapid diagnostic tests in Kédougou, Senegal.medRxiv [Preprint]. 2024 Dec 13:2024.12.12.24318945. doi: 10.1101/2024.12.12.24318945. medRxiv. 2024. Update in: Parasit Vectors. 2025 Jul 12;18(1):280. doi: 10.1186/s13071-025-06914-9. PMID: 39711730 Free PMC article. Updated. Preprint.
References
-
- WHO, Global Malaria Programme. How malaria RDTs work. 2021. https://www.who.int/teams/global-malaria-programme/case-management/diagn.... Accessed 19 Feb 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

