Estimating the effect of maternal viral load on perinatal and postnatal HIV transmission: a systematic review and meta-analysis
- PMID: 40652949
- PMCID: PMC12262163
- DOI: 10.1016/S0140-6736(25)00765-2
Estimating the effect of maternal viral load on perinatal and postnatal HIV transmission: a systematic review and meta-analysis
Abstract
Background: Although a growing body of evidence supports zero risk of sexual HIV transmission from a person with sustained virological suppression, known as U=U (undetectable equals untransmittable), data have been insufficient to determine whether this is also true for vertical HIV transmission. We conducted a systematic review and meta-analysis to quantify vertical transmission risk by maternal HIV viral load (mHVL) and to evaluate the applicability of U=U to perinatal and postnatal HIV transmission.
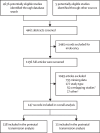
Methods: In this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, Cochrane Library, Cumulative Index of Nursing and Allied Health Literature, the WHO Global Health Library, and abstracts from the International AIDS Society Conference and the Conference on Retroviruses and Opportunistic Infections (2016-24) for studies published from Jan 1, 1989, to Dec 31, 2024, reporting the relationship between mHVL near birth (to estimate perinatal transmission risk by 6 weeks) or during breastfeeding (to estimate monthly postnatal transmission risk by mHVL within the past 6 months) and vertical transmission. We pooled risks of perinatal and postnatal transmission across prespecified mHVL categories. We also conducted comparative analyses to determine the adjusted relative risk (aRR) of transmission by mHVL using Poisson meta-regression. The protocol for this analysis is registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42019146768).
Findings: 147 studies were included in the analysis; 138 studies contributed to perinatal analyses and 13 studies contributed to postnatal analyses. Data on 82 723 mother-child pairs were included across all analyses. Pooled perinatal transmission risks were 0·2% (95% CI 0·2-0·3) with a mHVL of <50 copies per mL, 1·3% (1·0-1·7) with 50-999 copies per mL, and 5·1% (2·6-7·9) with ≥1000 copies per mL. aRRs of perinatal transmission were 6·3 (3·9-10·3) with a mHVL of 50-999 copies per mL and 22·5 (13·9-36·5) with ≥1000 copies per mL versus <50 copies per mL. In subgroup analyses, in five studies reporting on 4675 women receiving pre-conception antiretroviral therapy (ART) with a mHVL of <50 copies per mL near birth, there were zero (0%, 0·0-0·1) perinatal transmissions. Monthly postnatal transmission risks were 0·1% (0·0-0·4) with recent mHVL <50 copies per mL and 0·5% (0·1-1·8) with a mHVL of ≥50 copies per mL.
Interpretation: Perinatal transmission with a mHVL of <50 copies per mL is ≤0·2% overall. Zero transmissions were observed among women receiving ART before pregnancy with a mHVL of <50 copies per mL near birth, supporting U=U in pregnancy and birth. Postnatal transmission was very low-but not zero-among women with a recent mHVL of <50 copies per mL. Current data, largely from studies lacking frequent mHVL monitoring or modern first-line ART regimens, are insufficient to assess U=U during breastfeeding.
Funding: National Institutes of Health, WHO, and Massachusetts General Hospital.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests LM serves as a paid consultant to WHO on the safety of antiretroviral drugs in pregnancy. CMD and ALC received support from WHO to adapt the results from this analysis for presentation to the WHO HIV Guidelines Development Group. All other authors declare no competing interests.
Figures
References
-
- WHO Mother-to-child transmission of HIV. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/p...
-
- WHO Global guidance on criteria and processes for validation: elimination of mother-to-child transmission of HIV, syphilis and hepatitis B virus. Aug 4, 2022. https://www.who.int/publications/i/item/9789240039360
-
- UNAIDS The path that ends AIDS—2023 UNAIDS Global AIDS update. 2023. https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/202...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

