Fructose-induced synaptic and neuronal adaptations at neuropeptide Y/agouti-related peptide neurons
- PMID: 40653085
- PMCID: PMC12304765
- DOI: 10.1016/j.molmet.2025.102209
Fructose-induced synaptic and neuronal adaptations at neuropeptide Y/agouti-related peptide neurons
Abstract
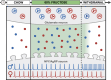
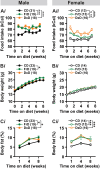
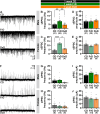
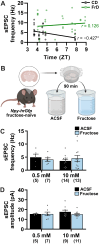
Fructose is a naturally-occurring sugar, consumed in excess as sweeteners, and is linked to the development of obesity. Fructose is consumed with glucose (dextrose) in added sugars, but while dextrose produces satiety, excessive fructose intake promotes hyperphagia through the brain. However, the neurological effects of dietary fructose are not clearly defined. We fed male and female mice standard chow, a 60% high fructose, or 60% high dextrose diet and found that fructose- and dextrose-fed mice ate more calories and gained more body fat despite increasing fat oxidation and energy expenditure. Furthermore, their metabolic syndromes were more prominent in male mice, who also developed glucose intolerance. To define the neurological effects underlying the obesogenic actions of fructose, we performed ex vivo patch-clamp recordings from orexigenic Neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons in the arcuate nucleus. Fructose feeding uniquely increased synaptic excitation at NPY/AgRP neurons, which remained elevated with sustained fructose exposure; this excitation may arise from glutamatergic neurons in the dorsomedial hypothalamic nucleus. Terminating fructose feeding reversed this synaptic excitation at male but not female NPY/AgRP neurons. Furthermore, chronic but not acute fructose feeding in male mice also irreversibly activated NPY/AgRP neurons even following fructose withdrawal. Interestingly, despite sex-dependent fructose-mediated plasticity at NPY/AgRP neurons, a prolonged fructose withdrawal increased innate fructose preference in both male and female mice. Taken together, these findings showed that fructose elicited synaptic and neuronal excitation at NPY/AgRP neurons that can be long-lasting. These actions are consistent with that seen during hunger and may thus promote hyperphagia in the expression of fructose-mediated obesity.
Keywords: Electrophysiology; Fructose; Hypothalamus; Obesity; Plasticity; Sugar.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. - PubMed
-
- Bray G.A. Commentary on: the fructose survival hypothesis as a mechanism for unifying the various obesity hypotheses. Obesity (Silver Spring) 2024;32:7–11. - PubMed
-
- Chiavaroli L., Cheung A., Ayoub-Charette A., Ahmed A., Lee D., Au-Yeung F., et al. Important food sources of fructose-containing sugars and adiposity: a systematic review and meta-analysis of controlled feeding trials. Am J Clin Nutr. 2023;117:741–765. - PubMed
-
- Sievenpiper J.L., de Souza R.J., Mirrahimi A., Yu M.E., Carleton A.J., Beyene J., et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304. - PubMed
-
- Rodgers A., Woodward A., Swinburn B., Dietz W.H. Prevalence trends tell us what did not precipitate the US obesity epidemic. Lancet Public Health. 2018;3:e162–e163. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

