Exploratory Study of CD10low Polymorphonuclear Leukocytes Preceding and Correlating With Postsurgical Inflammation
- PMID: 40653798
- PMCID: PMC12256972
- DOI: 10.1111/sji.70042
Exploratory Study of CD10low Polymorphonuclear Leukocytes Preceding and Correlating With Postsurgical Inflammation
Abstract
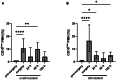
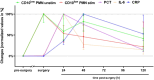
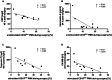
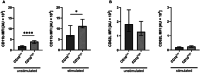
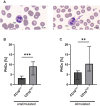
The lack of diagnostic and monitoring tools for postsurgical immunological complications such as systemic inflammation can contribute to a poor outcome despite modern intensive care. The need for reliable immune monitoring has been emphasised. Polymorphonuclear leukocytes (PMNs) play an important role in postsurgical inflammation. A subgroup of PMNs is particularly interesting, because they are released shortly after (iatrogenic) trauma: immature PMNs, characterised by, for example, their low CD10 expression. Therefore, we investigated the role of CD10low PMNs in a non-interventional exploratory study by including patients undergoing scheduled, highly standardised cardiac surgery with extracorporeal circulation. We were able to demonstrate that the number of CD10low PMNs released shortly after the beginning of surgery correlated with different fluid phase markers of inflammation and organ damage postsurgically. Among these parameters were CRP, IL-6, NGAL, CK-MB, and troponin-T. Noteworthy, the amount of CD10low PMNs increased as early as 24 h before these well-established markers, suggesting superiority of CD10low PMNs as an early diagnostic marker. Comparing CD10low immature PMNs with CD10high mature PMNs revealed potential involved mechanisms, including lower CD11b expression and a significant decrease in the formation of platelet-neutrophil complexes (PNCs) by CD10low PMNs. In conclusion, we propose CD10low PMNs as a potential early cellular biomarker to assess the postsurgical inflammatory response. In comparison to clinically established markers like CRP or IL-6 and scoring systems such as the SOFA-Score, CD10low PMNs reflect a potential candidate for future immune monitoring to determine the risk of excessive inflammation and organ impairment more rapidly.
© 2025 The Author(s). Scandinavian Journal of Immunology published by John Wiley & Sons Ltd on behalf of The Scandinavian Foundation for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wilharm A., Pflug A., Loos F., Sommerfeld O., Hofmann G. O., and Sauer S., “Causes of Death in the Seriously Injured—Why Do Severely Injured Patients Die Today?,” Zeitschrift für Orthopädie und Unfallchirurgie 161 (2023): 297–303. - PubMed
-
- Angus D. C. and van der Poll T., “Severe Sepsis and Septic Shock,” New England Journal of Medicine 369 (2013): 840–851. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

