Differential Modulation of Dopamine D2 Receptor on Somatostatin and Parvalbumin Interneurons in the CA1 Area of the Dorsal Hippocampus
- PMID: 40653805
- PMCID: PMC12256973
- DOI: 10.1111/ejn.70176
Differential Modulation of Dopamine D2 Receptor on Somatostatin and Parvalbumin Interneurons in the CA1 Area of the Dorsal Hippocampus
Abstract
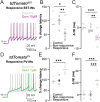
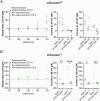
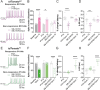
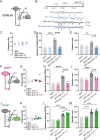
Spatiomolecular mapping of hippocampal dopamine D2 receptor neurons revealed that in addition to hilar mossy cells, hippocampal somatostatin interneurons and, to a lesser extent, parvalbumin interneurons expressed dopamine D2 receptor. However, the consequence of dopamine D2 receptor activation on hippocampal somatostatin interneurons and parvalbumin interneurons is unknown. By combining pharmacological approaches and patch-clamp recordings in organotypic hippocampal slices from control mice or mice lacking Drd2 selectively in somatostatin or parvalbumin interneurons, we found that dopamine D2 receptor activation increases excitability in somatostatin interneurons while it decreases it in parvalbumin interneurons in the CA1 area. These changes depend on voltage-gated K channels and rely on distinct intracellular pathways, involving the non-canonical β-arrestin-dependent pathway in somatostatin interneurons and the G protein-dependent pathway in parvalbumin interneurons. Finally, our study unveils that activation of dopamine D2 receptor in somatostatin interneurons modulates methacholine-induced rhythmic synaptic activity at 4-15 Hz.
Keywords: GABAergic cells; dopamine D2 receptor; hippocampus; intracellular signaling pathway; intrinsic excitability.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Computational modeling of ketamine-induced changes in gamma-band oscillations: The contribution of parvalbumin and somatostatin interneurons.PLoS Comput Biol. 2025 Jun 9;21(6):e1013118. doi: 10.1371/journal.pcbi.1013118. eCollection 2025 Jun. PLoS Comput Biol. 2025. PMID: 40489551 Free PMC article.
-
Excitatory Inputs Determine Phase-Locking Strength and Spike-Timing of CA1 Stratum Oriens/Alveus Parvalbumin and Somatostatin Interneurons during Intrinsically Generated Hippocampal Theta Rhythm.J Neurosci. 2016 Jun 22;36(25):6605-22. doi: 10.1523/JNEUROSCI.3951-13.2016. J Neurosci. 2016. PMID: 27335395 Free PMC article.
-
Synergistic deficits in parvalbumin interneurons and dopamine signaling drive ACC dysfunction in chronic pain.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2502558122. doi: 10.1073/pnas.2502558122. Epub 2025 Jun 26. Proc Natl Acad Sci U S A. 2025. PMID: 40569386
-
Dopamine D2-like receptors on conditioned and unconditioned fear: A systematic review of rodent pharmacological studies.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Aug 30;134:111080. doi: 10.1016/j.pnpbp.2024.111080. Epub 2024 Jun 29. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38950840
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

