Seasonal Transition in the Dominance of Photoautotrophic and Heterotrophic Protists in the Photic Layer of a Subtropical Marine Ecosystem
- PMID: 40654005
- PMCID: PMC12256934
- DOI: 10.1111/1758-2229.70126
Seasonal Transition in the Dominance of Photoautotrophic and Heterotrophic Protists in the Photic Layer of a Subtropical Marine Ecosystem
Abstract
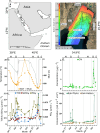
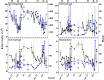
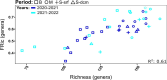
Protists are major functional players in the oceans. Time-resolved protist diversity and succession patterns remain poorly described in subtropical ecosystems, limiting current understanding of food web dynamics and responses to environmental changes in these major world-ocean regions. We used amplicon sequencing data and trait-based annotation to examine the seasonality of planktonic protists in the subtropical Gulf of Aqaba (Red Sea). Temperature and nutrients were the major drivers of succession. We detected marked seasonal shifts in protists. Heterotrophs, including diverse parasitic functional groups, dominated the warm, stratified oligotrophic period spanning spring and summer. By contrast, nutrient influx during deep convective mixing in winter triggered a shift to photoautotrophic communities dominated by a few genera of chlorophytes. Deeper winter mixing resulted in larger blooms at the onset of stratification dominated by diatoms, relative to chlorophytes that prevailed during shallower blooms. This result illustrates the impact of mixing depth on bloom formation and composition. Comparisons with oceanwide rDNA datasets indicate that the oligotrophic protist assemblages from the Gulf resemble those from warm, open oceans. This work provides a detailed assessment of the seasonal switch in dominant trophic functions in protists in phase with nutrient levels in a subtropical planktonic ecosystem.
Keywords: Red Sea; diversity; ecology; functional traits; photic layer; protists; subtropical.
© 2025 The Author(s). Environmental Microbiology Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Al‐Najjar, T. , Badran M. I., Richter C., Meyerhoefer M., and Sommer U.. 2007. “Seasonal Dynamics of Phytoplankton in the Gulf of Aqaba, Red Sea.” Hydrobiologia 579, no. 1: 69–83. 10.1007/s10750-006-0365-z. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

