Irisin Prevents the Effects of Simulated Microgravity on Bone and Muscle Differentiation Markers
- PMID: 40654332
- PMCID: PMC12246387
- DOI: 10.1096/fba.2025-00085
Irisin Prevents the Effects of Simulated Microgravity on Bone and Muscle Differentiation Markers
Abstract
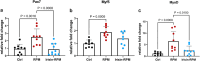
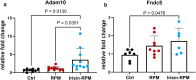
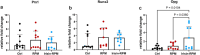
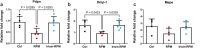
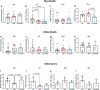
Microgravity exposure affects both tissues and cells, and, in this regard, one of the most affected targets is the skeletal muscle system due to the significant loss of bone and muscle mass leading to osteoporosis and sarcopenia, respectively. Several efforts are underway to counteract the effects of microgravity, and recent studies on irisin, a myokine with anabolic effects on the musculoskeletal system, have shown promising results. Due to the practical challenges of conducting experiments in actual microgravity, different devices generating a simulated microgravity condition on Earth have been developed. Here, we exposed myoblasts, osteoblasts, osteocytes to a random position machine (RPM) for five days to assess microgravity effect on the expression of key differentiation factors in cells untreated or treated with irisin. In myoblasts (C2C12), exposure to RPM led to increased expression of early myogenesis maker genes Pax7 (p = 0.0016), Myf5 (p = 0.0005) and MyoD (p = 0.0009). Irisin treatment in the last 8 h of RPM cultures prevented these increases by returning Pax7 (p = 0.0008) and MyoD (p = 0.01) to control values, and only partially Myf5. In bone cells, exposure to RPM for 5 days showed no effect in osteoblasts (MC3T3) but decreased the expression of Pdpn (p = 0.0285) and Dmp-1 (p = 0.0423) genes in osteocytes (MLO-Y4). Irisin treatment completely prevented the decline in Pdpn (p = 0.293) and Dmp-1 (p = 0.0339) levels. Overall, our data showed that the impact of RPM exposure keeps myoblasts and osteocytes in a proliferative state, and irisin treatment restores them to their baseline biological condition, suggesting that irisin can counteract the changes induced by simulated microgravity.
Keywords: bone; irisin; microgravity; muscle; myoblasts; osteoblasts; osteocytes; random position machine.
© 2025 The Author(s). FASEB BioAdvances published by the Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Convertino V. A., “Physiological Adaptations to Weightlessness: Effects on Exercise and Work Performance,” Exercise and Sport Sciences Reviews 18 (1990): 119–166. - PubMed
-
- Tilton F. E., Degioanni J. J., and Schneider V. S., “Long‐Term Follow‐Up of Skylab Bone Demineralization,” Aviation, Space, and Environmental Medicine 51, no. 11 (1980): 1209–1213. - PubMed
LinkOut - more resources
Full Text Sources
