SRSF7 promotes pulmonary fibrosis through regulating PKM alternative splicing in lung fibroblasts
- PMID: 40654335
- PMCID: PMC12254822
- DOI: 10.1016/j.apsb.2025.04.017
SRSF7 promotes pulmonary fibrosis through regulating PKM alternative splicing in lung fibroblasts
Abstract
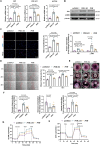
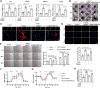
Idiopathic pulmonary fibrosis (IPF), a chronic interstitial lung disease, is characterized by aberrant wound healing, excessive scarring and the formation of myofibroblastic foci. Although the role of alternative splicing (AS) in the pathogenesis of organ fibrosis has garnered increasing attention, its specific contribution to pulmonary fibrosis remains incompletely understood. In this study, we identified an up-regulation of serine/arginine-rich splicing factor 7 (SRSF7) in lung fibroblasts derived from IPF patients and a bleomycin (BLM)-induced mouse model, and further characterized its functional role in both human fetal lung fibroblasts and mice. We demonstrated that enhanced expression of Srsf7 in mice spontaneously induced alveolar collagen accumulation. Mechanistically, we investigated alternative splicing events and revealed that SRSF7 modulates the alternative splicing of pyruvate kinase (PKM), leading to metabolic dysregulation and fibroblast activation. In vivo studies showed that fibroblast-specific knockout of Srsf7 in conditional knockout mice conferred resistance to bleomycin-induced pulmonary fibrosis. Importantly, through drug screening, we identified lomitapide as a novel modulator of SRSF7, which effectively mitigated experimental pulmonary fibrosis. Collectively, our findings elucidate a molecular pathway by which SRSF7 drives fibroblast metabolic dysregulation and propose a potential therapeutic strategy for pulmonary fibrosis.
Keywords: Alternative splicing; Drug screening; Fibroblasts; IPF; Metabolism; PKM; SRSF7; Splicing factor.
© 2025 The Authors.
Conflict of interest statement
The authors declared no conflict of interest.
Figures







References
-
- Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

