This is a preprint.
Chromosomal instability shapes the tumor microenvironment of esophageal adenocarcinoma via a cGAS-chemokine-myeloid axis
- PMID: 40654626
- PMCID: PMC12248057
- DOI: 10.1101/2025.05.06.652454
Chromosomal instability shapes the tumor microenvironment of esophageal adenocarcinoma via a cGAS-chemokine-myeloid axis
Abstract
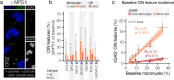
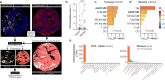
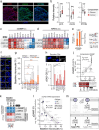
Chromosomal instability (CIN), a characteristic feature of esophageal adenocarcinoma (EAC), drives tumor aggressiveness and therapy resistance, presenting an intractable problem in cancer treatment. CIN leads to constitutive stimulation of the innate immune cGAS-STING pathway, which has been typically linked to anti-tumor immunity. However, despite the high CIN burden in EAC, the cGAS-STING pathway remains largely intact. To address this paradox, we developed novel esophageal cancer models, including a CIN-isogenic model, discovering myeloid-attracting chemokines - with the chemokine CXCL8 (IL-8) as a prominent hit - as conserved CIN-driven targets in EAC. Using high-resolution multiplexed immunofluorescence microscopy, we quantified the extent of ongoing cGAS-activating CIN in human EAC tumors by measuring cGAS-positive micronuclei in tumor cells, validated by orthogonal whole-genome sequencing-based CIN metrics. By coupling in situ CIN assessment with single-nucleus RNA sequencing and multiplex immunophenotypic profiling, we found tumor cell-intrinsic innate immune activation and intratumoral myeloid cell inflammation as phenotypic consequences of CIN in EAC. Additionally, we identified increased tumor cell-intrinsic CXCL8 expression in CINhigh EAC, accounting for the inflammatory tumor microenvironment. Using a novel signature of CIN, termed CINMN, which captures ongoing CIN-associated gene expression, we confirm poor patient outcomes in CINhigh tumors with signs of aberrantly rewired cGAS-STING pathway signaling. Together, our findings help explain the counterintuitive maintenance and expression of cGAS-STING pathway components in aggressive, CINhigh tumors and emphasize the need to understand the contribution of CIN to the shaping of a pro-tumor immune landscape. Therapeutic strategies aimed at disrupting the cGAS-driven inflammation axis may be instrumental in improving patient outcomes in this aggressive cancer.
Keywords: Chromosomal instability; cGAS; chemokine; esophagogastric cancer; myeloid; tumor microenvironment.
Figures

















References
-
- Shah M. A. et al. Improving outcomes in patients with oesophageal cancer. Nat Rev Clin Oncol 20, 390–407 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
