This is a preprint.
Advanced Cardiac Organoid Model for Studying Doxorubicin-Induced Cardiotoxicity
- PMID: 40654805
- PMCID: PMC12247883
- DOI: 10.1101/2025.05.02.651878
Advanced Cardiac Organoid Model for Studying Doxorubicin-Induced Cardiotoxicity
Update in
-
Advanced cardiac organoid model for studying doxorubicin-induced cardiotoxicity.Toxicol Sci. 2025 Nov 1;208(1):95-103. doi: 10.1093/toxsci/kfaf115. Toxicol Sci. 2025. PMID: 40796330
Abstract
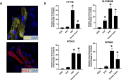
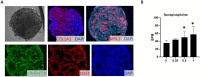
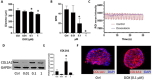
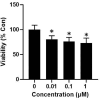
Cardiac organoids provide an in vitro platform for studying heart disease mechanisms and drug responses. However, a major limitation is the immaturity of cardiomyocytes, restricting their ability to mimic adult cardiac physiology. Additionally, the inadequacy of commonly used extracellular matrices (ECM), which fail to replicate the biochemical and mechanical properties of natural heart tissue, poses significant challenges. Consequently, structural integrity in cardiac organoids is impaired. Moreover, scalability remains an obstacle, as conventional ECM substitutes hinder mass production of organoids for high-throughput toxicology screening. To overcome these challenges, we developed an advanced model promoting fibroblast-driven ECM self-secretion, enabling physiologically relevant tissue architecture and function. Using the ECM-free, mature cardiomyocyte-integrated organoid model, we investigated the cardiotoxicity of doxorubicin, a widely used chemotherapeutic agent known to impair cardiac function. Cardiomyocytes derived from induced pluripotent stem cells were characterized for maturity by immunostaining for cTNT and MYL2 alongside gene expression analysis. Organoids treated with doxorubicin showed reduced size and increased collagen deposition. These structural changes correlated with functional impairments, including decreased contraction rate and disrupted synchronous beating. In 2D culture, exposure to doxorubicin induced fibroblast activation, promoted endothelial-to-mesenchymal transition in endothelial cells, and triggered cytotoxic effects in cardiomyocytes. This study highlights the importance of ECM remodeling in advancing cardiac organoid models and demonstrates its potential for more accurate cardiotoxicity assessment. Addressing these limitations enhances the physiological relevance of cardiac organoid systems for drug safety assessment and cardiac disease modeling.
Keywords: cardiac organoid; cardiotoxicity; chemotherapy; pluripotent stem cells.
Conflict of interest statement
Declaration of conflicting interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Abriel H., and Kass R. S.. 2005. ‘Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins’, Trends Cardiovasc Med, 15: 35–40. - PubMed
-
- Benjanuwattra J., Siri-Angkul N., Chattipakorn S. C., and Chattipakorn N.. 2020. ‘Doxorubicin and its proarrhythmic effects: A comprehensive review of the evidence from experimental and clinical studies’, Pharmacol Res, 151: 104542. - PubMed
-
- Dark N., Cosson M. V., Tsansizi L. I., Owen T. J., Ferraro E., Francis A. J., Tsai S., Bouissou C., Weston A., Collinson L., Abi-Gerges N., Miller P. E., MacLeod K. T., Ehler E., Mitter R., Harding S. E., Smith J. C., and Bernardo A. S.. 2023. ‘Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells’, Cell Rep Methods, 3: 100456. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
