This is a preprint.
PTEN regulates starburst amacrine cell dendrite morphology during development
- PMID: 40654823
- PMCID: PMC12248082
- DOI: 10.1101/2025.05.08.652956
PTEN regulates starburst amacrine cell dendrite morphology during development
Abstract
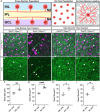
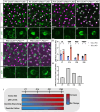
Neurons are subject to extensive developmental regulation to ensure precise subtype-specific morphologies that are intimately tied to their function. Starburst amacrine cells (SACs) in the mammalian retina have a highly stereotyped, radially symmetric dendritic arbor that is essential for their role in direction-selective circuits in the retina. We show that PTEN, the primary negative regulator of the PI3K-AKT-mTOR pathway that is highly implicated in neurodevelopmental disorders, regulates SAC morphology in a cell-autonomous manner. Pten-deficient SACs show a nearly twofold increase in the number of dendritic branches, while other morphological properties remain largely unchanged. These morphological changes arise late in SAC development after dendrite development is largely complete and persist into adulthood. Mechanistically, excessive dendritic branching appears to arise from dysregulated mTOR activity. Despite this dramatic increase in dendritic branches, Pten-deficient SACs maintain a normal population number, organization of synaptic outputs, and intact direction-selectivity in the retina. Collectively, these results show that PTEN is essential for the normal development of highly stereotyped neuronal morphology.
Figures





Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
AMIGO1 Promotes Axon Growth and Territory Matching in the Retina.J Neurosci. 2022 Mar 30;42(13):2678-2689. doi: 10.1523/JNEUROSCI.1164-21.2022. Epub 2022 Feb 15. J Neurosci. 2022. PMID: 35169021 Free PMC article.
-
A cross-species analysis of neuroanatomical covariance sex differences in humans and mice.Biol Sex Differ. 2025 Jul 1;16(1):47. doi: 10.1186/s13293-025-00728-1. Biol Sex Differ. 2025. PMID: 40598550 Free PMC article.
-
The PI3K/Akt/mTOR Signaling Pathway in Triple-Negative Breast Cancer: A Resistance Pathway and a Prime Target for Targeted Therapies.Cancers (Basel). 2025 Jul 3;17(13):2232. doi: 10.3390/cancers17132232. Cancers (Basel). 2025. PMID: 40647529 Free PMC article. Review.
References
-
- Lefebvre J.L., Sanes J.R., and Kay J.N., Development of dendritic form and function. Annu Rev Cell Dev Biol, 2015. 31: p. 741–77. - PubMed
-
- Zeng H. and Sanes J.R., Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci, 2017. 18(9): p. 530–546. - PubMed
-
- Lefebvre J.L., Molecular mechanisms that mediate dendrite morphogenesis. Curr Top Dev Biol, 2021. 142: p. 233–282. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
