This is a preprint.
In Vivo Directed Evolution of an Ultra-Fast Rubisco from a Semi-Anaerobic Environment Imparts Oxygen Resistance
- PMID: 40654885
- PMCID: PMC12247655
- DOI: 10.1101/2025.02.17.638297
In Vivo Directed Evolution of an Ultra-Fast Rubisco from a Semi-Anaerobic Environment Imparts Oxygen Resistance
Update in
-
In vivo directed evolution of an ultrafast Rubisco from a semianaerobic environment imparts oxygen resistance.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2505083122. doi: 10.1073/pnas.2505083122. Epub 2025 Jun 30. Proc Natl Acad Sci U S A. 2025. PMID: 40587785 Free PMC article.
Abstract
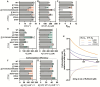
Carbon dioxide (CO2) assimilation by the enzyme Ribulose-1,5-bisphosphate Carboxylase/Oxygenase (Rubisco) underpins biomass accumulation in photosynthetic bacteria and eukaryotes. Despite its pivotal role, Rubisco has a slow carboxylation rate and is competitively inhibited by oxygen (O2). These traits impose limitations on photosynthetic efficiency, making Rubisco a compelling target for improvement. Interest in Form II Rubisco from Gallionellaceae bacteria, which comprise a dimer or hexamer of large subunits, arises from their nearly 5-fold higher than the average Rubisco enzyme. As well as having a fast (25.8 s -1 at 25 °C), we show that Gallionellaceae Rubisco (GWS1B) is extremely sensitive to O2 inhibition, consistent with its evolution under semi-anaerobic environments. We therefore used a novel in vivo mutagenesis-mediated screening pipeline to evolve GWS1B over six rounds under oxygenic selection, identifying three catalytic point mutants with improved ambient carboxylation efficiency; Thr-29-Ala (T29A), Glu-40-Lys (E40K) and Arg-337-Cys (R337C). Full kinetic characterization showed that each substitution enhanced the CO2 affinity of GWS1B under oxygenic conditions by subduing oxygen affinity, leading to 25% (E40K), 11% (T29A) and 8% (R337C) enhancements in carboxylation efficiency under ambient O2 at 25 °C. By contrast, under the near anaerobic natural environment of Gallionellaceae, the carboxylation efficiency of each mutant was impaired ~16%. These findings demonstrate the efficacy of artificial directed evolution to access novel regions of catalytic space in Rubisco.
Figures



References
-
- Prywes N., Phillips N. R., Tuck O. T., Valentin-Alvarado L. E., Savage D. F., Rubisco function, evolution, and engineering. Annu. Rev. Biochem. 92, 385–410 (2023). - PubMed
-
- Tabita F. R., Satagopan S., Hanson T. E., Kreel N. E., Scott S. S., Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 59, 1515–1524 (2008). - PubMed
-
- Schulz L. et al. , Evolution of increased complexity and specificity at the dawn of form I Rubiscos. Science 378, 155–160 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
