Targeting phospholipase PLAG-15 promotes healthy aging in C. elegans via lysosomal-related genes
- PMID: 40655094
- PMCID: PMC12246578
- DOI: 10.1016/j.isci.2025.112880
Targeting phospholipase PLAG-15 promotes healthy aging in C. elegans via lysosomal-related genes
Abstract
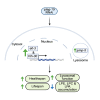
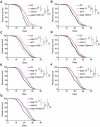
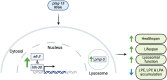
Complex lipid metabolism plays a crucial role in regulating aging. We recently discovered that the phospholipid bis(monoacylglycero)phosphate (BMP) increases in aged human muscles and many mouse tissues. The phospholipase PLA2G15 is reportedly involved in BMP synthesis, however, its specific role in aging remains unknown. To elucidate the role of PLA2G15 in aging, we used Caenorhabditis elegans as a model. When silencing plag-15, the predicted worm orthologue of PLA2G15, we observed improved healthspan and lifespan extension. Semi-targeted lipidomics highlighted that instead of changes related to BMP, plag-15 RNAi led to lower levels of lysophosphatidic acid, lysophosphatidylcholine, and lysophosphatidylethanolamine. Transcriptome-guided epistasis experiments identified that the lifespan extension of plag-15 RNAi worms is regulated by transcription factors hlh-30 and elt-3, and lysosomal vitamin B12 transporter pmp-5 (human TFEB, GATA, and ABCD4 respectively). Overall, we conclude that targeting phospholipid remodeling through plag-15 could be a promising strategy to promote healthy aging.
Keywords: Lipidomics; Molecular physiology; Transcriptomics.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

