Virus-host interaction mechanisms in interferon therapy for hepatitis B virus infection: recent advances
- PMID: 40655152
- PMCID: PMC12245691
- DOI: 10.3389/fimmu.2025.1603544
Virus-host interaction mechanisms in interferon therapy for hepatitis B virus infection: recent advances
Abstract
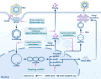
Chronic hepatitis B virus (HBV) infection has been implicated in the development of liver diseases, such as hepatitis, fibrosis, cirrhosis, and cancer, which negatively affect the patients' quality of life and impacts a high economic strain on patients. The persistence of covalently closed circular DNA (cccDNA) allows the propagation of the infection, and no drug have been developed to completely eliminate cccDNA. The available drugs for chronic hepatitis B (CHB) are classified into nucleos(t)ide analogs (NAs) and interferon-α (IFN-α)/pegylated interferon α (Peg-IFN-α). However, these treatments do not effectively eradicate hepatitis B surface antigen (HBsAg) and their clinical efficacy is limited. The potential of IFN-based clinical cure is increasingly attracting interest from hepatologists, but the therapeutic outcomes of this intervention are suboptimal and some of them are associated with various complications. Although several novel antiviral drugs are being investigated, however, achieving a clinical cure based on monotherapy is currently challenging. The efficacy of IFN therapy is influenced by host and viral factors. This article provides a comprehensive review of host-related factors that affect the IFN therapy for CHB. A thorough understanding and management of these host-related factors will enhance the efficacy of interferon treatment, minimize adverse reactions, improve patient tolerance, and thereby increasing the clinical cure rate of hepatitis B.
Keywords: antiviral treatment; chronic hepatitis b; host factors; interferon-α; virus-host interactions.
Copyright © 2025 Zhang, Lou, Zhu, Wang, Gong and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association, Group of Infectious Diseases, Chinese Pediatric Society Research progress on clinical antiviral treatment of chronic hepatitis B in children. Zhonghua Gan Zang Bing Za Zhi. (2024) 32(5):435–48. doi: 10.3760/cma.j.cn501113-20240415-00206 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

