Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons
- PMID: 40655413
- PMCID: PMC12245631
- DOI: 10.21769/BioProtoc.5367
Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons
Abstract
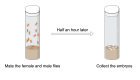
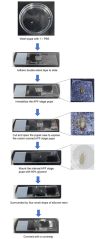
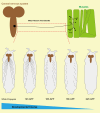
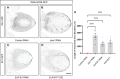
Over the lifespan of an individual, brain function requires adjustments in response to environmental changes and learning experiences. During early development, neurons overproduce neurite branches, and neuronal pruning removes the unnecessary neurite branches to make a more accurate neural circuit. Drosophila motoneurons prune their intermediate axon bundles rather than the terminal neuromuscular junction (NMJ) by degeneration, which provides a unique advantage for studying axon pruning. The pruning process of motor axon bundles can be directly analyzed by real-time imaging, and this protocol provides a straightforward method for monitoring the developmental process of Drosophila motor neurons using live cell imaging. Key features • Long-range projecting axon bundles of Drosophila motor neurons extending from soma on the ventral nerve cord (VNC) undergo degeneration rather than retraction during metamorphosis. • The pruning process of motor axon bundles can be directly observed by real-time live-cell imaging. • The complete clearance of axon bundles occurs approximately 22 h after pupal formation (22 h APF). • Mushroom body (MB) γ neuron axon pruning regulatory genes are conserved for motor neurons.
Keywords: Axonal pruning; Drosophila melanogaster; Live cell imaging; Motor neurons; Neurodevelopment.
©Copyright : © 2025 The Authors; This is an open access article under the CC BY-NC license.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
-
- Tsai N. P., Wilkerson J. R., Guo W., Maksimova M. A., DeMartino G. N., Cowan C. W. and Huber K. M.(2012). Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95. Cell. 151(7): 1581 1594 1594. 10.1016/j.cell .2012.11.040 - DOI - PMC - PubMed
-
- Tang G., Gudsnuk K., Kuo S. H., Cotrina M. L., Rosoklija G., Sosunov A., Sonders M. S., Kanter E., Castagna C., Yamamoto A., et al.(2014). Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron. 83(5): 1131 1143 1143. 10.1016/j.neuron .2014.07.040 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

