Demethylzeylasteral inhibits proliferation and metastasis of osteosarcoma cells by modulating the PI3K/AKT/Autophagy pathways
- PMID: 40655907
- PMCID: PMC12246626
- DOI: 10.1016/j.jbo.2025.100699
Demethylzeylasteral inhibits proliferation and metastasis of osteosarcoma cells by modulating the PI3K/AKT/Autophagy pathways
Abstract
Background: Osteosarcoma (OS) remains a highly aggressive malignancy with limited treatment options, necessitating the discovery of novel therapeutic agents. Demethylzeylasteral (DEM), a compound previously shown to exert anti-tumor properties in several malignancies, has not been sufficiently explored for its potential in OS treatment.
Purpose: This study focused on the anti-tumor properties of DEM on OS cells as well as the potential mechanisms.
Methods: OS cell lines (MG63 and 143B) were exposed to varying concentrations of DEM, followed by assessment of diverse cell functions. RNA sequencing was implemented to identify the molecular pathways affected by DEM exposure. The mechanistic underpinnings of DEM's action were also studied via a series of assays. Additionally, the therapeutic potential was validated utilizing xenograft models.
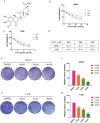
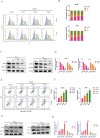
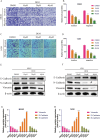
Results: DEM evidently repressed OS cell proliferation in a dose- and time-dependent fashion, arrested cells in G2/M phase, and facilitated apoptosis through the modulation of the BCL2/BAX ratio. Furthermore, DEM suppressed cell migration and invasion by reversing EMT-related protein expression. RNA sequencing revealed that DEM primarily affected autophagy-related pathways, particularly through the PI3K/AKT signaling. DEM treatment led to an elevation in ROS generation and enhanced autophagic activity, as demonstrated by elevated LC3B puncta formation and autophagy-related protein expression. In vivo, DEM effectively suppressed tumor growth while showing a favorable safety profile.
Conclusion: This study provides comprehensive evidence that DEM exerts potent anti-tumor properties in OS via the PI3K/AKT pathway, highlighting the significance of DEM as a therapeutic candidate for OS.
Keywords: Autophagy; Demethylzeylasteral; Osteosarcoma; PI3K/AKT pathway.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Quinacrine induces autophagy via the Dlg5/AKT pathway to inhibit osteosarcoma cell proliferation and suppresses migration and invasion through the Dlg5/Girdin pathway.Phytomedicine. 2025 Sep;145:156981. doi: 10.1016/j.phymed.2025.156981. Epub 2025 Jun 12. Phytomedicine. 2025. PMID: 40541124
-
Caveolin-1 inhibits the proliferation and invasion of lung adenocarcinoma via EGFR degradation.Sci Rep. 2025 Jul 1;15(1):21654. doi: 10.1038/s41598-025-05259-8. Sci Rep. 2025. PMID: 40594106 Free PMC article.
-
DDTC-Cu(I) inhibits human osteosarcoma cells growth by repressing MET/PI3K/AKT signaling pathway.Sci Rep. 2025 Jul 1;15(1):21780. doi: 10.1038/s41598-025-06748-6. Sci Rep. 2025. PMID: 40594499 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Arora R.S., et al. The contrasting age-incidence patterns of bone tumours in teenagers and young adults: implications for aetiology. Int. J. Cancer. 2012;131(7):1678–1685. - PubMed
-
- Valery P.C., Laversanne M., Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26(8):1127–1139. - PubMed
-
- Nat. Rev. Dis. Primers. 2022;8(1):76. - PubMed
-
- Anderson M.E. Update on survival in Osteosarcoma. Orthop. Clin. North Am. 2016;47(1):283–292. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

