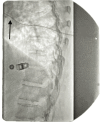
Wet Tap-Induced Spinal Cord Stimulator Trial Failure in Failed Back Surgery Syndrome: A Case Report Highlighting Intrathecal Drug Delivery for Treatment-Resistant Pain
- PMID: 40656251
- PMCID: PMC12252154
- DOI: 10.7759/cureus.85793
Wet Tap-Induced Spinal Cord Stimulator Trial Failure in Failed Back Surgery Syndrome: A Case Report Highlighting Intrathecal Drug Delivery for Treatment-Resistant Pain
Abstract
A 59-year-old female with failed back surgery syndrome (FBSS) following multiple lumbar surgeries underwent a spinal cord stimulator (SCS) trial for chronic pain management. However, the trial failed due to severe nausea, vomiting, and postural headaches secondary to unintentional dural puncture (wet tap) during lead placement. This resulted in cerebrospinal fluid (CSF) leakage, low-pressure headaches, and subsequent trial intolerance, necessitating early lead removal. Given her failed response to conservative treatments, neuropathic medications, and systemic opioids, an intrathecal pump (ITP) trial was pursued, yielding 90% pain relief and leading to permanent pump implantation. This case highlights the impact of procedural complications on neuromodulation outcomes and the role of intrathecal drug delivery as an alternative in patients who cannot tolerate SCS.
Keywords: failed back surgery syndrome (fbss); intrathecal pump therapy; neuromodulation therapies; spinal cord stimulation (scs); unintentional dural puncture.
Copyright © 2025, Patel et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Spinal cord stimulation for cancer-related pain in adults.Cochrane Database Syst Rev. 2015 Jun 29;2015(6):CD009389. doi: 10.1002/14651858.CD009389.pub3. Cochrane Database Syst Rev. 2015. PMID: 26121600 Free PMC article.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.Health Technol Assess. 2009 Mar;13(17):iii, ix-x, 1-154. doi: 10.3310/hta13170. Health Technol Assess. 2009. PMID: 19331797
-
Spinal cord stimulation for cancer-related pain in adults.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD009389. doi: 10.1002/14651858.CD009389.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Jun 29;(6):CD009389. doi: 10.1002/14651858.CD009389.pub3. PMID: 23450600 Updated.
-
Cervical Spinal Cord Stimulator Malfunction Secondary to Lead Fracture.Pain Med Case Rep. 2025 Jun;9(3):151-156. Pain Med Case Rep. 2025. PMID: 40608365
-
Epidural spread of surgical site infection from spinal cord stimulation trial.Pain Manag. 2024 Jun 2;14(5-6):235-240. doi: 10.1080/17581869.2024.2373044. Epub 2024 Jul 8. Pain Manag. 2024. PMID: 38973311 Free PMC article.
References
-
- The epidemiology and economic consequences of pain. Henschke N, Kamper SJ, Maher CG. Mayo Clin Proc. 2015;90:139–147. - PubMed
-
- Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. North RB, Campbell JN, James CS, et al. https://pubmed.ncbi.nlm.nih.gov/1831546/ Neurosurgery. 1991;28:685–691. - PubMed
-
- Orhurhu VJ, Chu R, Gill J. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Failed back surgery syndrome. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous