Camrelizumab plus SOX chemotherapy as adjuvant therapy for pathological stage III gastric or gastroesophageal junction adenocarcinoma: a prospective, multicenter, single-arm, phase II trial
- PMID: 40656603
- PMCID: PMC12254632
- DOI: 10.1177/17588359251355781
Camrelizumab plus SOX chemotherapy as adjuvant therapy for pathological stage III gastric or gastroesophageal junction adenocarcinoma: a prospective, multicenter, single-arm, phase II trial
Abstract
Background: Immune checkpoint inhibitors have shown promising results in the treatment of advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma.
Objectives: To evaluate the safety and efficacy of camrelizumab combined with S-1 plus oxaliplatin chemotherapy in the postoperative setting for pathological stage III (pStage III) G/GEJ adenocarcinoma.
Design: This study was a prospective, multicenter, single-arm, phase II trial.
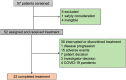
Methods: Patients with pStage III G/GEJ adenocarcinoma were enrolled, receiving camrelizumab (200 mg), followed by oxaliplatin (130 mg/m2), both administered on day 1 of each 21-day cycle, and tegafur-gimeracil-oteracil potassium capsules (40-60 mg, twice daily) for 2 weeks, followed by a 7-day rest period. A total of eight treatment cycles were planned. The primary endpoints were the incidence of any-grade and grade 3 or 4 adverse events. The secondary endpoints were disease-free survival (DFS), overall survival (OS), and treatment completion rate.
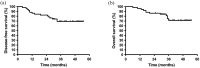
Results: Between September 2020 and March 2023, 52 patients were enrolled at three medical centers in Zhejiang Province, China. All 52 patients (100%) experienced treatment-related adverse events (TRAEs). Grade 3 or higher TRAEs were reported in 35 patients (67.3%). Events occurring in >10% of the patients included decreased neutrophil count, neutropenia, leukopenia, thrombocytopenia, and anemia. Eleven (21.2%) patients experienced TRAEs that led to the interruption or discontinuation of camrelizumab, including rash (1), hypothyroidism (1), hyperkalemia (1), interstitial pneumonia (2), cystitis or urethritis (2), and reactive cutaneous capillary endothelial proliferation (4). The actual 2-year DFS and OS rates were 82.4% and 86.3%, respectively, whereas the estimated 3-year DFS and OS rates were 69.1% and 71.3%, respectively.
Conclusion: Camrelizumab combined with chemotherapy has a manageable and tolerable safety profile as an adjuvant treatment for pStage III G/GEJ adenocarcinoma. However, careful patient selection is necessary to identify patients most likely to benefit from combination therapy.
Trial registration: ClinicalTrials.gov registry: NCT04515615; Date of registration: August 14, 2020; Weblink: https://clinicaltrials.gov/study/NCT04515615.
Keywords: SOX regimen; camrelizumab; gastric or gastroesophageal junction adenocarcinoma; survival; treatment-related adverse events.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): final report of a randomised, open-label, phase 3 trial.Lancet Oncol. 2025 Mar;26(3):312-319. doi: 10.1016/S1470-2045(24)00676-4. Epub 2025 Feb 11. Lancet Oncol. 2025. PMID: 39952264 Clinical Trial.
-
Adjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy for stage III gastric or gastro-oesophageal junction cancer after gastrectomy with D2 or more extensive lymph-node dissection (ATTRACTION-5): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial.Lancet Gastroenterol Hepatol. 2024 Aug;9(8):705-717. doi: 10.1016/S2468-1253(24)00156-0. Epub 2024 Jun 18. Lancet Gastroenterol Hepatol. 2024. PMID: 38906161 Clinical Trial.
-
Postoperative adjuvant chemotherapy in rectal cancer operated for cure.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004078. doi: 10.1002/14651858.CD004078.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419291 Free PMC article.
-
Pathologic Response of Phase III Study: Perioperative Camrelizumab Plus Rivoceranib and Chemotherapy Versus Chemotherapy for Locally Advanced Gastric Cancer (DRAGON IV/CAP 05).J Clin Oncol. 2025 Feb;43(4):464-474. doi: 10.1200/JCO.24.00795. Epub 2024 Oct 9. J Clin Oncol. 2025. PMID: 39383487 Free PMC article. Clinical Trial.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74(3): 229–263. - PubMed
-
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357(18): 1810–1820. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous