Enhanced and proficient chitosan membranes embedded with polyaniline-TiO2 core-shell nanocomposites for fuel-cell hydrogen storage
- PMID: 40656876
- PMCID: PMC12253973
- DOI: 10.55730/1300-0527.3730
Enhanced and proficient chitosan membranes embedded with polyaniline-TiO2 core-shell nanocomposites for fuel-cell hydrogen storage
Abstract
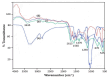
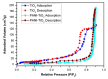
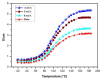
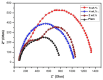
This study investigates the preparation and properties of aniline polymerized in situ onto a nanosized TiO2 surface to form core-shell nanoparticles at ambient temperatures. The in situ polymerization of aniline to polyaniline (PANI), in conjunction with the utilization of an anionic surfactant, was employed in this investigation. The prepared PANI-TiO2 core-shell nanoparticles were integrated with chitosan at a gravimetric ratio and cast as core-shell nanocomposite membranes. The nanocomposites were subjected to structural analysis using Fourier transform infrared spectroscopy and X-ray diffraction patterns. The surface morphologies of the PANI and its nanocomposites were analyzed using scanning electron microscopy. Direct current conductivity studies revealed three discrete tiers of conductivity intrinsic to a semiconductor material. The nanocomposite, comprising a chitosan membrane embedded with 4 wt.% PANI-TiO2, demonstrated peak direct current conductivity of 5.7 S/cm. The properties of the core-shell nanocomposite membranes could be elucidated using cyclic voltammetry, a technique that allowed for the observation of redox peaks occurring at 0.94 V and 0.25 V. The presence of both peaks was due to the redox transition of the prepared nanocomposite membranes from a semiconducting to a conductive state. At room temperature, the hydrogen absorption capacity was approximately 4.5 wt.%, but when the temperature was raised to 65 °C, it doubled to about 7.5 wt.%. In comparison to other nanocomposites, the 4 wt.% PANI-TiO2 core-shell embedded chitosan membrane exhibited significantly higher absorption capacity of 10.5 wt.%.
Keywords: Metal oxide nanoparticles; core-shell; direct current conductivity; hydrogen storage; polyaniline.
© TÜBİTAK.
Conflict of interest statement
Conflicts of interest: The authors declare no conflicts of interest.
Figures








References
-
- Apostolou D. Xydis GA literature review on hydrogen refuelling stations and infrastructure. Current status and future prospects. Renewable and Sustainable Energy Reviews. 2019;113:109–292. doi: 10.1016/j.rser.2019.109292. - DOI
-
- Dincer I, Acar C. Smart energy solutions with hydrogen options. International Journal of Hydrogen Energy. 2018;43(18):8579–8599. doi: 10.1016/j.ijhydene.2018.03.120. - DOI
-
- Khan N, Kalair E, Abas N, Kalair AR, Kalair A. Energy transition from molecules to atoms and photons. Engineering Science and Technology an International Journal. 2019;22(1):185–214. doi: 10.1016/j.jestch.2018.05.002. - DOI
-
- To WM, Lee PKC. Energy consumption and economic development in Hong Kong, China. Energies. 2017;10(11):1883. doi: 10.3390/en10111883. - DOI
-
- Mohsin M, Kamran HW, Nawaz MA, Hussain MS, Dahri AS. Assessing the impact of transition from nonrenewable to renewable energy consumption on economic growth-environmental nexus from developing Asian economies. Journal of Environmental Management. 2021;284:111–999. doi: 10.1016/j.jenvman.2021.111999. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
