Dispersive micro solid phase extraction of cadmium on MIL-53(Al)@BaTiO3 nanocomposite from seafood samples
- PMID: 40656883
- PMCID: PMC12253969
- DOI: 10.55730/1300-0527.3733
Dispersive micro solid phase extraction of cadmium on MIL-53(Al)@BaTiO3 nanocomposite from seafood samples
Abstract
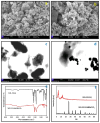
This study's main goal was to produce a user-friendly dispersive micro solid phase extraction (dmSPE) technique with a MIL-53(Al)@BaTiO3 nanocomposite for the extraction and preconcentration of cadmium (Cd) in various seafood matrices, followed by using high-resolution continuum source flame atomic absorption spectrometry (HR-CS-FAAS). The MIL-53(Al)@BaTiO3 nanocomposite was synthesized and characterized using a range of techniques, including Fourier-transform infrared spectroscopy, field emission scanning electron microscopy, scanning transmission electron microscopy, X-ray diffraction, and Brunauer-Emmett-Teller analysis. The dmSPE technique involved the dispersion of the MIL-53(Al)@BaTiO3 material in the sample solution, followed by its separation from the sample matrix. The optimized method exhibited a linear range of 3.6-250 μg L-1, a limit of detection (LOD) of 1.2 μg L-1, and a preconcentration factor of 80. Two different certified reference materials were used to ensure the validation of developed method. The method was applied to different seafood samples.
Keywords: Cadmium; micro solid phase extraction; nanocomposite; seafoods.
© TÜBİTAK.
Figures
Similar articles
-
A novel MIP electrochemical sensor based on a CuFe2O4NPs@rGO nanocomposite and its application in breast milk samples for the determination of fipronil.Anal Methods. 2025 Jul 3;17(26):5508-5518. doi: 10.1039/d5ay00911a. Anal Methods. 2025. PMID: 40557723
-
Development of BaSrTiO3 nanomaterial based dispersive solid phase microextraction method for cadmium determination in thyme samples using flame atomic absorption spectrometry.Sci Rep. 2025 Jul 7;15(1):24168. doi: 10.1038/s41598-025-08464-7. Sci Rep. 2025. PMID: 40624162 Free PMC article.
-
Exploring a green and precise approach based on graphene oxide-cobalt ferrite nanocomposite for detection and quantification of vitamin B9 and antibacterial assessment.Sci Rep. 2025 Jul 4;15(1):23964. doi: 10.1038/s41598-025-09893-0. Sci Rep. 2025. PMID: 40615669 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
References
-
- Dahaji RRM, Moghimi A, Shahbaz H, Faraji H, Azizinejad F. Preparation of modified multiwalled carbon nanotubes (L-Arg-CS/MWCNTs-COOH/Fe3O4) as sorbent for dispersive solid phase extraction of Cu(II), Pb(II) and Cd(II) in wastewaer samples. Revue Roumaine De Chimie. 2023;68:495–505. doi: 10.33224/rrch.2023.68.10-12.01. - DOI
-
- Kurnaz Yetim N, Berberoğlu EA, Aslan N, Koç MM, Özcan C. Sonochemical removal of Pb (II) ions from the water medium using Bi2S3 nanostructres. International Journal of Environmental Analytical Chemistry. 2024;104:3586–3601. doi: 10.1080/03067319.2022.2088288. - DOI
-
- Conchado-Amado P, Sánchez-Piñero J, Moreda-Piñeiro J, Turnes-Carou I, López-Mahía P, et al. Ultra-trace mercury determination in seawater after vortex-assisted liquid-liquid micro-extraction. Spectrochimica Acta Part B: Atomic Spectroscopy. 2023;204:106683. doi: 10.1016/j.sab.2023.106683. - DOI
-
- Khan M, Ozalp O, Khan M, Soylak M. Fe3O4-Ti3AlC2 max phase impregnated with 2-(5-Bromo-2-pyridylazo-5-(diethylamino) phenol for magnetic solid phase extraction of Cadmium, lead and cobalt from water and food samples. Journal of Molecular Liquids. 2022;368:120685. doi: 10.1016/j.molliq.2022.120685. - DOI
LinkOut - more resources
Full Text Sources
