Beyond cost: a value-based framework for biosimilar procurement in emerging markets - the Thai experience
- PMID: 40656965
- PMCID: PMC12247093
- DOI: 10.1080/20523211.2025.2526827
Beyond cost: a value-based framework for biosimilar procurement in emerging markets - the Thai experience
Abstract
Background: Biosimilars offer potential for increased access to critical biological therapies in emerging-markets like Thailand. However, making procurement decisions for these complex molecules requires considering multiple factors beyond cost. This study aimed to develop and test a context-specific, value-based procurement framework for biosimilars in Thailand using Multi-Criteria Decision Analysis (MCDA).
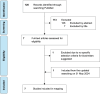
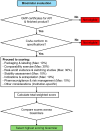
Methods: This study employed a mixed-methods approach, combining a literature review with expert consensus workshops. The literature review identified existing biosimilar selection criteria, which were mapped onto the Most Economically Advantageous Tender (MEAT) Value-Based Procurement (VBP) framework. Two expert consensus workshops were conducted to develop and refine a Thai-specific MCDA tool. The initial framework underwent a feasibility study in five hospitals across Thailand, with feedback incorporated into the refined version.
Results: The literature review yielded seven relevant studies, predominantly from established markets. The expert workshops produced a tailored MCDA tool with seven key criteria: Quality Assessment, Stability Assessment, Comparability Exercise, Indications and Extrapolation, Pharmacovigilance and Risk Management, Real-World Evidence and Switching Studies, and Other Considerations. Each criterion was weighted based on its perceived importance in the Thai context. The feasibility study resulted in refinements, such as adjusting criterion weights and incorporating flexibility for hospital-specific priorities.
Conclusion: This study presents a comprehensive, context-specific MCDA tool for biosimilar procurement in Thailand. By balancing scientific rigour with practical considerations, this framework has the potential to optimise biosimilar selection, enhancing access to vital biological therapies while ensuring quality and cost-effectiveness. The approach taken to develop this framework could serve as an initiative for other emerging markets seeking to improve their biosimilar procurement strategies.
Keywords: Biosimilars; Thailand; emerging markets; multi-criteria decision analysis; value-based procurement.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Butani, D., Faradiba, D., Dabak, S. V., Isaranuwatchai, W., Huang-Ku, E., Pachanee, K., Soboon, B., Culyer, A. J., & Teerawattananon, Y. (2023). Expanding access to high-cost medicines under the universal health coverage scheme in Thailand: Review of current practices and recommendations. Journal of Pharmaceutical Policy and Practice, 16(1), 138. 10.1186/s40545-023-00643-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources