Greening of Monocyclic N- and O‑Azole Synthesis through Sonochemistry
- PMID: 40657106
- PMCID: PMC12242661
- DOI: 10.1021/acsomega.5c02214
Greening of Monocyclic N- and O‑Azole Synthesis through Sonochemistry
Abstract
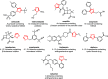
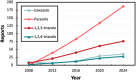
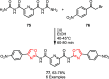
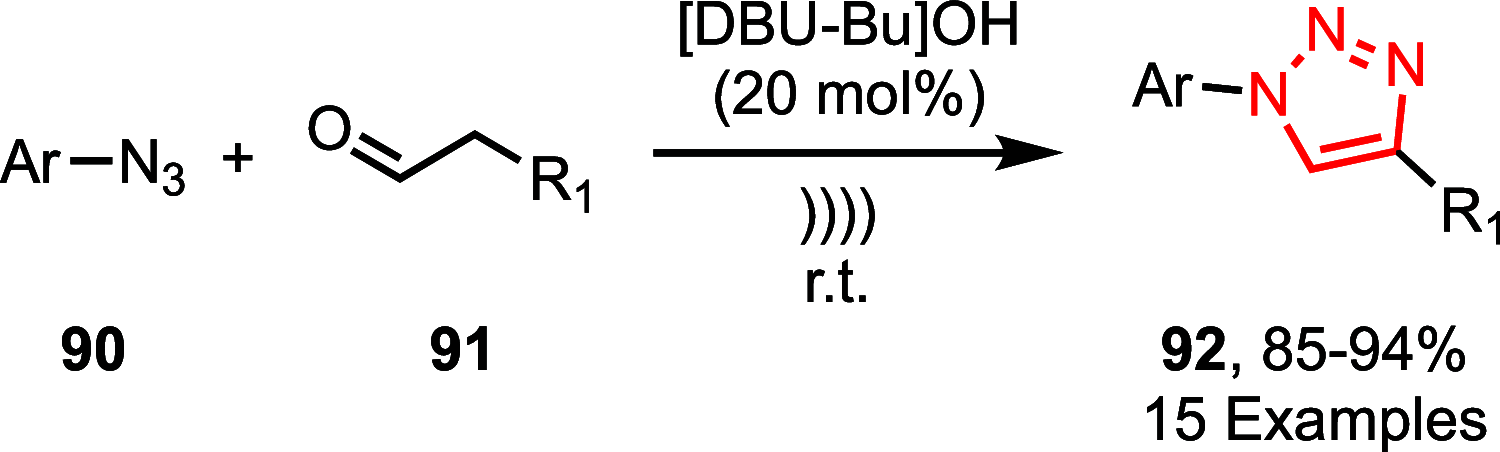
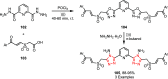
Sonochemistry is a fascinating branch of chemistry that applies sound waves in the ultrasonic frequency range to facilitate chemical processes. Although this technique is powerful, it remains underutilized in synthetic chemistry. By leveraging mechanisms of acoustic cavitation, sonochemistry initiates and enhances reactions, offering advantages such as cost-effectiveness, environmental sustainability, and improved reaction efficiency. Significantly, product yields in ring-closing reactions can be enhanced through sonochemistry. In certain cases, sonochemistry may also lead to altered product distributions. In this narrative review, we delve into the realm of N- and O-azoles, noting that the existing literature on sonochemistry as a synthetic method constitutes only about a tenth of all reports in this area. However, it is noteworthy that nearly all studies indicate improved outcomes when employing sonochemistry relative to traditional methods. As a green and sustainable synthetic approach, sonochemistry plays a crucial role in the synthesis of important heterocycles, many of which have significant pharmaceutical applications. This review covers synthetic reports including reaction conditions, solvents, catalysts, and reagents, spanning the past three decades, with an emphasis on contributions from 2020 through early 2025.
© 2025 The Authors. Published by American Chemical Society.
Figures

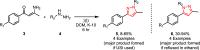


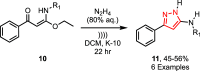












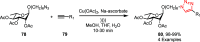

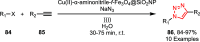


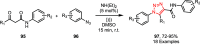



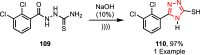


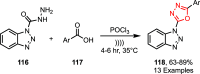



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Kardani H., Vaghani H., Patel P.. Green Waves in Medicinal Chemistry: Ultrasound Driven Multicomponent Reactions for Sustainable Synthesis of Biologically Active Molecules. Phosphorus Sulfur Silicon Relat. Elem. 2024;199:876–897. doi: 10.1080/10426507.2024.2430539. - DOI
-
- Lévêque, J.-M. ; Cravotto, G. ; Delattre, F. ; Cintas, P. . Organic Sonochemistry: Challenges and Perspectives for the 21st Century; Springer International Publishing: Cham, 2018. 10.1007/978-3-319-98554-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
