Natural Product Bruceine A from (L.) Merr. as a Potential LDLR Inhibitor That Facilitates Antiviral Effect
- PMID: 40657109
- PMCID: PMC12242671
- DOI: 10.1021/acsomega.5c02956
Natural Product Bruceine A from (L.) Merr. as a Potential LDLR Inhibitor That Facilitates Antiviral Effect
Abstract
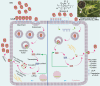
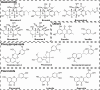
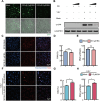
Low-density lipoprotein receptor (LDLR), which serves as one of the most major entry receptors for many viruses in both human and mouse cells, plays a vital role in virus infection. However, there are no effective small molecules available to inhibit LDLR expression and exhibit antiviral effects. Here, we screened Bruceine A (BA), a natural product derived from the major constituents in (L.) Merr. which inhibited vesicular stomatitis virus (VSV) infection in vitro. Mechanistically, BA blocked viral adsorption and internalization, facilitating antiviral effects through lysosome-mediated degradation of LDLR. Genetic knockdown of Ldlr exhibited strong antiviral effects. To the best of our knowledge, BA is the first LDLR-selective inhibitor, and our findings reveal that BA may serve as a potent and broad-spectrum virus entry inhibitor based on LDLR entry receptor.
© 2025 The Authors. Published by American Chemical Society.
Figures

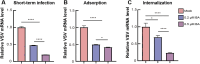

Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Interventions for prevention of herpes simplex labialis (cold sores on the lips).Cochrane Database Syst Rev. 2015 Aug 7;2015(8):CD010095. doi: 10.1002/14651858.CD010095.pub2. Cochrane Database Syst Rev. 2015. PMID: 26252373 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Vitamin C for asthma and exercise-induced bronchoconstriction.Cochrane Database Syst Rev. 2013 Oct 23;2013(10):CD010391. doi: 10.1002/14651858.CD010391.pub2. Cochrane Database Syst Rev. 2013. PMID: 24154977 Free PMC article.
References
-
- Yang Y., Ding T., Cong Y., Luo X., Liu C., Gong T., Zhao M., Zheng X., Li C., Zhang Y., Zhou J., Ni C., Zhang X., Ji Z., Wu T., Yang S., Zhou Q., Wu D., Gong X., Zheng Q., Li X.. Interferon-induced transmembrane protein-1 competitively blocks Ephrin receptor A2-mediated Epstein-Barr virus entry into epithelial cells. Nat. Microbiol. 2024;9(5):1256–1270. doi: 10.1038/s41564-024-01659-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
