Intravitreal Administration of Adalimumab-Loaded Poly(Lactic-co-Glycolic Acid) Nanoparticles: Effects on Biodistribution and Pharmacokinetics
- PMID: 40657186
- PMCID: PMC12245000
- DOI: 10.1002/smsc.202400494
Intravitreal Administration of Adalimumab-Loaded Poly(Lactic-co-Glycolic Acid) Nanoparticles: Effects on Biodistribution and Pharmacokinetics
Abstract
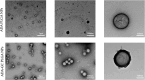
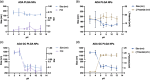
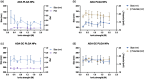
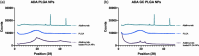
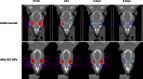
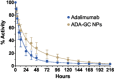
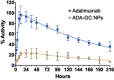
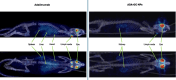
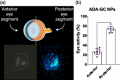
Adalimumab, a monoclonal antibody used for treating inflammatory diseases, including eye diseases, faces challenges in biodistribution and targeted delivery. Nanoparticle (NP)-based drug delivery systems have shown promise in enhancing the pharmacokinetic profiles of biologic drugs. This study aims to develop, and characterize intravitreal adalimumab-loaded poly(lactic-co-glycolic acid) (PLGA) NPs to improve antibody distribution and therapeutic efficacy. Characterization studies, morphological examination, and quantitative, stability, and physical properties are conducted. In vitro release kinetics are assessed using a dialysis membrane method. In vivo biodistribution is studied in rats after intravitreal administration by Positron Emission Tomography/Computed Tomography imaging. The optimized NPs were spherical (around 300 nm) with a surface charge of about -20 mV. Encapsulation efficiency and drug loading reach values close to 100%. Stability studies showed minimal changes in particle size and drug content. In vitro release showed a biphasic pattern with an initial burst release followed by sustained release. Safety studies indicated no significant cytotoxicity or adverse effects. The adalimumab-loaded PLGA NPs demonstrate favorable physicochemical characteristics, stability, and release profiles. In vivo distribution revealed a change in the antibody's distribution pattern after intravitreal administration via NPs encapsulation. These findings suggest the potential for enhanced therapeutic outcomes and warrant further investigation in disease-specific models to explore the clinical potential of this NP-based delivery system.
Keywords: adalimumab; controlled releases; in vivo distribution and pharmacokinetics; nanoparticles; poly(lactic‐co‐glycolic acid).
© 2024 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
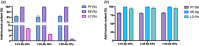







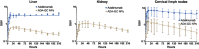
References
-
- Varela‐Fernández R., Bendicho‐Lavilla C., Martin‐Pastor M., Herrero Vanrell R., Lema‐Gesto M. I., González‐Barcia M., Otero‐Espinar F. J., Int. J. Pharm. 2022, 616, 121504. - PubMed
-
- Kishimoto T. K., Ferrari J. D., Lamothe R. A., Kolte P. N., Griset A. P., O'neil C., Chan V., Browning E., Chalishazar A., Kuhlman W., Fu F., Viseux N., Altreuter D. H., Johnston L., Maldonado R. A., Nat. Nanotechnol. 2016, 11 890. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
