Radioresistant triple-negative breast cancer cells release β-catenin containing extracellular vesicles to promote cancer stem cell activity of bystanders
- PMID: 40657369
- PMCID: PMC12243990
- DOI: 10.7150/jca.111555
Radioresistant triple-negative breast cancer cells release β-catenin containing extracellular vesicles to promote cancer stem cell activity of bystanders
Abstract
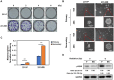
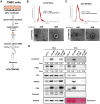
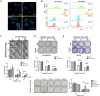
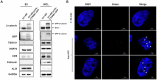
Background: Triple-negative breast cancer (TNBC) frequently develops radioresistance, yet the mechanisms remain incompletely elucidated. This study is the first to investigate how β-catenin, transported by extracellular vesicles (EVs) from radioresistant TNBC cells, promotes radioresistance and enhances cancer stem cell (CSC) activity in recipient TNBC cells, offering a novel mechanism distinct from prior EV-related findings in other cancers. Methods and Results: A radioresistant cell line (231-RR) was developed from MDA-MB-231 cells, and EVs were isolated for characterization. EVs from 231-RR cells decreased radiosensitivity in parental MDA-MB-231 and two other TNBC cell lines (MDA-MB-468 and Hs578T), as shown by clonogenic assay. These EVs also enhanced CSC activity in MDA-MB-231 and Hs578T cells, demonstrated through primary and secondary mammosphere formation. The effects were nullified when using EVs from 231-RR cells treated with the EV secretion inhibitor GW4869. 231-RR-derived EVs showed elevated β-catenin levels and increased active β-catenin and stemness proteins (c-Myc, OCT4, SOX2) in recipient TNBC cells. The β-catenin inhibitor CCT-031374 prevented EV-mediated enhancement of radioresistance and CSC activity. Public data analysis from breast cancer patients revealed post-radiotherapy upregulation of the β-catenin pathway, with elevated CTNNB1, MYC, and CD44 expression, alongside reduced CDKN2A and CDH1 levels, supporting clinical relevance. Conclusions: This study uniquely demonstrates that EVs from radioresistant TNBC cells transfer β-catenin to confer radioresistance and enhance CSC activity in recipient cells, a mechanism not previously reported in TNBC. These findings suggest the potential of EV-β-catenin derived as a novel biomarker for predicting radiotherapy outcomes and recurrence risk in TNBC patients, pending development of sensitive detection methods.
Keywords: cancer stem cells; extracellular vesicles; radioresistance; triple negative breast cancer; β-catenin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
4T1 breast cancer cells exposed to extracellular vesicles from MDA-MB-231 cells stimulated with bisphenol A increase the growth of mammary tumors and metastasis in female Balb/cJ mice.Mol Cell Endocrinol. 2025 Aug 7:112641. doi: 10.1016/j.mce.2025.112641. Online ahead of print. Mol Cell Endocrinol. 2025. PMID: 40782997
-
Intercellular communication between extracellular vesicles from conditioned macrophages and breast cancer cells drives endocrine therapy resistance.Front Cell Dev Biol. 2025 Jun 3;13:1548724. doi: 10.3389/fcell.2025.1548724. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40567500 Free PMC article.
-
The R-RAS2 GTPase is a signaling hub in triple-negative breast cancer cell metabolism and metastatic behavior.J Hematol Oncol. 2025 Apr 12;18(1):41. doi: 10.1186/s13045-025-01693-3. J Hematol Oncol. 2025. PMID: 40221767 Free PMC article.
-
Platinum-based chemotherapy for early triple-negative breast cancer.Cochrane Database Syst Rev. 2023 Sep 8;9(9):CD014805. doi: 10.1002/14651858.CD014805.pub2. Cochrane Database Syst Rev. 2023. PMID: 37681577 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247–69. - PubMed
-
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J. et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

