Gabapentin's effect on human dorsal root ganglia: Donor-specific electrophysiological and transcriptomic profiles
- PMID: 40657722
- PMCID: PMC12368406
- DOI: 10.1177/17448069251361712
Gabapentin's effect on human dorsal root ganglia: Donor-specific electrophysiological and transcriptomic profiles
Abstract
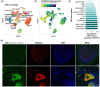
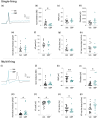
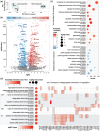
Neuropathic pain affects approximately 10% of the adult population and is commonly treated with gabapentin (GBP), a repurposed anticonvulsant drug. Despite its widespread use, GBP's effectiveness varies significantly among patients, highlighting the need to better understand its functional and molecular impacts on human nociceptors. Here we characterized the electrophysiological and transcriptomic effects of GBP on primary neurons derived from the dorsal root ganglia (DRGs) of ethically consented human donors. Using patch-clamp electrophysiology, we demonstrated that GBP treatment reduced neuronal excitability, with more pronounced effects in multi-firing vs. single-firing neurons. Notably, significant donor-specific variability was observed in electrophysiological responsiveness to GBP treatment in vitro. RNA sequencing of DRG tissue from the donor that was more responsive to GBP revealed differences in transcriptome-wide expression of genes associated with ion transport, synaptic transmission, inflammation, and immune response. Cross-transcriptomic analyses further showed that GBP treatment counteracted these alterations, rescuing aberrant gene expression at the pathway level and for several key genes. This study provides a comprehensive electrophysiological and transcriptomic profile of the effects of GBP on human DRG neurons. These findings enhance our understanding of GBP's mechanistic actions on peripheral sensory neurons and could help optimize its use for managing neuropathic pain.
Keywords: Gabapentin; hDRG; ion channel; sensory neurons; α2δ1.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Magrinelli F, Zanette G, Tamburin S. Neuropathic pain: diagnosis and treatment. Pract Neurol 2013; 13: 292–307. - PubMed
-
- Alles SRA, Smith PA. Etiology and pharmacology of neuropathic pain. Pharmacol Rev 2018; 70: 315–347. - PubMed
-
- Jay GW, Barkin RL. Neuropathic pain: etiology, pathophysiology, mechanisms, and evaluations. Dis Mon 2014; 60: 6–47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

