Sacituzumab Govitecan Population Pharmacokinetics: Updated Analyses Using HR+/HER2- Metastatic Breast Cancer Data From the Phase 3 TROPiCS-02 Trial
- PMID: 40657894
- PMCID: PMC12257498
- DOI: 10.1111/cts.70291
Sacituzumab Govitecan Population Pharmacokinetics: Updated Analyses Using HR+/HER2- Metastatic Breast Cancer Data From the Phase 3 TROPiCS-02 Trial
Abstract
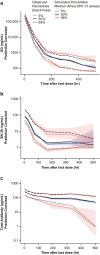
Sacituzumab govitecan (SG) is an antibody-drug conjugate composed of a Trop-2-directed antibody coupled to SN-38. SG is approved in multiple countries for pretreated metastatic triple-negative breast cancer (mTNBC) and hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) mBC. Three previously developed population pharmacokinetic (PopPK) models for SG, free SN-38, and total antibody (tAB) in patients with mTNBC or other solid tumors were externally validated using data from 260 patients with HR+/HER2- mBC from TROPiCS-02 (NCT03901339). Pharmacokinetic parameters were re-estimated using data from 789 patients with HR+/HER2- mBC, mTNBC, or other solid tumors from three studies-TROPiCS-02, ASCENT (NCT02574455), and IMMU-132-01 (NCT01631552). Previously developed PopPK models adequately described the data from TROPiCS-02. Typical parameter estimates based on combined dataset for clearance and steady-state volume of distribution were 0.128 L/h and 3.58 L for SG and 0.0155 L/h and 4.29 L for tAB, respectively. The pharmacokinetics of the three analytes (SG, free SN-38, and tAB) in participants with HR+/HER2- mBC were consistent with those observed in mTNBC and other tumor types. The analyses confirmed mild-to-moderate renal impairment, mild hepatic impairment, age, tumor type (based on limited data in non-breast cancer tumor types), baseline albumin level, UGT1A1 genotype, or Trop-2 expression did not have a clinically relevant impact on the exposure of the three analytes across populations. These findings support that the SG dosing regimen of 10 mg/kg on Days 1 and 8 of 21-day cycles is adequate for patients with HR+/HER2- mBC.
Keywords: cancers; clinical trials; metastasis; modeling; phase III; population pharmacokinetics; tumors.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Abhishek G. Sathe is an employee of and holds stocks in Gilead Sciences, Inc. Aksana K. Jones was an employee of Gilead Sciences, Inc. at the time this research was conducted and holds stocks in Gilead Sciences, Inc. Paul M. Diderichsen is an employee of Certara, and received support for the current manuscript and consulting fees from Gilead Sciences, Inc. Xiaohui Wang reports nothing to disclose. Peter Chang is an employee of and holds stock in Certara, and received support for the current manuscript from Gilead Sciences, Inc. Wendy Verret is an employee of and holds stocks in Gilead Sciences, Inc. Sandhya Girish is an employee of and holds stocks in Gilead Sciences, Inc.
Figures


References
-
- National Cancer Institute , “Cancer Stat Facts: Female Breast Cancer Subtypes,” accessed June 6, 2024, https://seer.cancer.gov/statfacts/html/breast‐subtypes.html.
-
- National Comprehensive Cancer Network , “Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V4.2024,” © National Comprehensive Cancer Network Inc., 2024. All rights reserved, accessed July 3, 2024. To view the most recent and complete version of the guideline, go online to, NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. NCCN=National Comprehensive Cancer Network® (NCCN®).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

