Histone variant H2A.Z cooperates with EBNA1 to maintain Epstein-Barr virus latent epigenome
- PMID: 40657913
- PMCID: PMC12345143
- DOI: 10.1128/mbio.00302-25
Histone variant H2A.Z cooperates with EBNA1 to maintain Epstein-Barr virus latent epigenome
Abstract
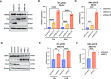
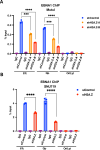
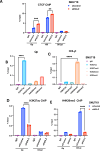
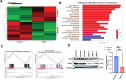
Chromatin structure plays a central role in the regulation of Epstein-Barr virus (EBV) latency. The histone variant H2A.Z.1 has been implicated in chromatin structures associated with the initiation of transcription and DNA replication. Here, we investigate the functional role of H2AZ.1 in the regulation of EBV chromatin, gene expression, and copy number during latent infection. We found that H2A.Z.1 is highly enriched near EBNA1-binding sites at the origin of plasmid replication (oriP) and the transcriptional start site for the EBNA1 gene (Qp), and to a lesser extent with transcriptionally active CTCF binding sites on the EBV genomes in both Mutu I Burkitt lymphoma (BL) and SNU719 EBV-associated gastric carcinoma (EBVaGC) cell lines. RNA-interference depletion of H2A.Z.1 resulted in the reactivation of viral lytic genes (ZTA and EAD) and increased viral DNA copy numbers in both MutuI and SNU719 cells. H2A.Z depletion also led to a decrease in EBNA1 binding to oriP and Qp, on the viral episome as well as on oriP plasmids independently of other viral genes and genomes. H2A.Z.1 depletion also reduced peaks of H3K27ac and H4K20me3 at regulatory elements in the EBV genome. In the cellular genome, H2A.Z.1 colocalized with only a subset of EBNA1 binding sites, and H2A.Z.1 depletion reduced EBNA1 binding to these sites and altered the transcription of genes associated with myc targets and mTORC1 signaling. Taken together, these findings indicate that H2A.Z.1 cooperates with EBNA1 to regulate chromatin structures important for epigenetic programming of the latent episome.IMPORTANCECellular factors that maintain viral latency are of fundamental importance. We have found that the cellular histone variant H2A.Z functions in cooperation with the Epstein-Barr virus (EBV) latency maintenance protein EBNA1 to establish a stable epigenome and prevent lytic cycle reactivation during latency. We show that H2A.Z localizes near EBNA1-binding sites on the viral and host genomes, facilitates EBNA1 binding at these sites, and is required for epigenetic programming of viral episomes. H2A.Z depletion perturbed cMyc and mTORC1 pathways that have been implicated in the control of EBV latency. These findings suggest that H2A.Z is an essential constituent of EBV chromatin required for EBNA1 binding and stable maintenance of EBV latency.
Keywords: Epstein-Barr virus; epigenetic; herpesviruses; histones; latency.
Conflict of interest statement
Paul Lieberman is a founder and advisor of Vironika, LLC, and holds patents on a small molecule inhibitor of EBNA1. All other authors have no conflicts to disclose.
Figures




Update of
-
Histone Variant H2A.Z Cooperates with EBNA1 to Maintain Epstein-Barr Virus Latent Epigenome.bioRxiv [Preprint]. 2025 Jan 29:2025.01.28.635203. doi: 10.1101/2025.01.28.635203. bioRxiv. 2025. Update in: mBio. 2025 Aug 13;16(8):e0030225. doi: 10.1128/mbio.00302-25. PMID: 39975074 Free PMC article. Updated. Preprint.
Similar articles
-
Histone Variant H2A.Z Cooperates with EBNA1 to Maintain Epstein-Barr Virus Latent Epigenome.bioRxiv [Preprint]. 2025 Jan 29:2025.01.28.635203. doi: 10.1101/2025.01.28.635203. bioRxiv. 2025. Update in: mBio. 2025 Aug 13;16(8):e0030225. doi: 10.1128/mbio.00302-25. PMID: 39975074 Free PMC article. Updated. Preprint.
-
HCF1 and OCT2 Cooperate with EBNA1 To Enhance OriP-Dependent Transcription and Episome Maintenance of Latent Epstein-Barr Virus.J Virol. 2016 May 12;90(11):5353-5367. doi: 10.1128/JVI.00239-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009953 Free PMC article.
-
USP7 Inhibitors Destabilize EBNA1 and Suppress Epstein-Barr Virus Tumorigenesis.J Med Virol. 2025 Jan;97(1):e70168. doi: 10.1002/jmv.70168. J Med Virol. 2025. PMID: 39821265 Free PMC article.
-
EBV Latency Programs: Molecular and Epigenetic Regulation and Its Role in Disease Pathogenesis.J Med Virol. 2025 Jul;97(7):e70501. doi: 10.1002/jmv.70501. J Med Virol. 2025. PMID: 40685972 Free PMC article. Review.
-
Evaluating the Clinical Utility of Epstein-Barr Virus Antibodies as Biomarkers in Multiple Sclerosis: A Systematic Review.Mult Scler Relat Disord. 2024 Apr;84:105410. doi: 10.1016/j.msard.2023.105410. Epub 2023 Dec 30. Mult Scler Relat Disord. 2024. PMID: 38401201
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
