Therapeutic and prophylactic effects of Qipian on COPD in mice: the role of lung and gut microbiota
- PMID: 40657918
- PMCID: PMC12327850
- DOI: 10.1128/spectrum.01969-24
Therapeutic and prophylactic effects of Qipian on COPD in mice: the role of lung and gut microbiota
Abstract
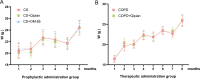
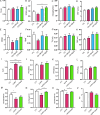
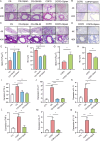
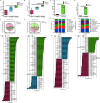
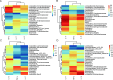
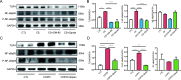
The objective of this study was to investigate the therapeutic and prophylactic effects of the mixed extract of Staphylococcus and Neisseria tablets (Qipian) on chronic obstructive pulmonary disease (COPD) in mice and its relationship with lung and gut microbiota. C57BL/6 mice were divided into two models (prophylactic versus therapeutic administration). In the model of prophylactic administration, the mice were exposed to cigarette smoke (CS) for 16 weeks and divided into CS+Qipian group, CS+OM-85 group, and CS control group. Compared to the CS control group, mice in both CS+Qipian and CS+OM-85 groups had improved lung function, reduced pulmonary bullae and inflammatory cell infiltration, reduced collagen deposition around the bronchi, and decreased levels of inflammatory cytokines in the lung tissue. Qipian and OM-85 significantly increased the diversity of gut and lung microbiota and the abundance of Bacteroidetes, Bacteroidales, and Lactobacillus, as well as inhibited the toll-like receptor 4 (TLR4)/nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) pathway. There were no significant differences in the above parameters between the CS+OM-85 group and the CS+Qipian group. In the therapeutic administration model, the mice were exposed to CS for 24 weeks, and the results were consistent with those of the prophylactic administration model. Qipian administration has both preventive and therapeutic effects on COPD possibly by regulating the microbiota dysbiosis through the gut-lung axis, inhibiting the activation of the TLR4/NF-κB pathway, reducing inflammation, and improving lung function.IMPORTANCEGut and lung microbiota are associated with the development and progression of chronic obstructive pulmonary disease (COPD). Qipian treatment significantly increased the diversity of gut and lung microbiota and the abundance of Bacteroidetes, Bacteroidales, and Lactobacillus. In addition, the toll-like receptor 4/nuclear factor kappa-light-chain enhancer of activated B cells pathway mediated by the gut-lung axis may play an essential role in preventing and treating the pathogenesis of COPD, which allows for reduced inflammation and improvement of lung function.
Keywords: Staphylococcus and Neisseria tablet; chronic obstructive pulmonary disease; gut microbiota; lung microbiota; toll-like receptor 4.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, et al. 2018. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet 391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

