Japanese clinical guidelines for neovascular age-related macular degeneration
- PMID: 40658332
- PMCID: PMC12339655
- DOI: 10.1007/s10384-025-01240-0
Japanese clinical guidelines for neovascular age-related macular degeneration
Abstract
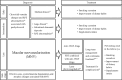
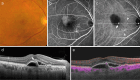
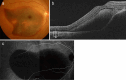
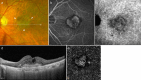
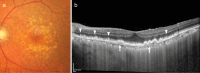
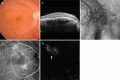
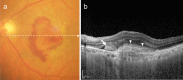
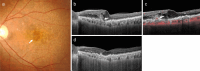
Recent advances in imaging technology and increased options of pharmaceutical therapy require that guidelines on the diagnostic criteria and treatment of neovascular age-related macular degeneration (AMD) be updated at regular intervals. These guidelines aim to standardize the management of neovascular AMD based on the latest understanding of its pathophysiology, advancements in diagnostic imaging modalities, and treatment options. The key updates include: (1) a revision of terminology and stage classification, adopting the AMD classifications of atrophic and neovascular, and adding end-stage AMD to the existing early, intermediate, and late stages; (2) the inclusion of pachychoroid in addition to drusen in the initial pathophysiology and pathogenic background; (3) diagnostic criteria defined by the presence of macular neovascularization based on multimodal imaging, including optical coherence tomography (OCT) and OCT angiography; (4) assessment of disease activity based on OCT; and (5) treatment guidance, including prophylaxis and low vision care as well as loading and maintenance phases by use of anti-vascular endothelial growth factor therapy and adjunctive therapies. We hope that these guidelines will be useful for those working in clinical practice in Japan, in other Asian countries, and in countries outside Asia.
Keywords: Guideline; Macular neovascularization; Neovascular AMD; Pachychoroid; Polypoidal choroidal vasculopathy.
© 2025. The Author(s).
Conflict of interest statement
Conflicts of interest: T. Iida, Grants (NIDEK, Topcon, Santen, Novartis, Senju, Alcon, HOYA, AMO), Consulting fees (Bayer, Chugai, Novartis, Boehringer Ingelheim, Janssen, Senju, Kyowa Kirin), Payment or honoraria for lectures (Bayer, Novartis, Alcon, Santen, Senju, Topcon, Chugai, Canon, Nidek, Otsuka, Nikon), Patents planned, issued or pending (Topcon) ; F. Gomi, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Novartis, Chugai, Santen, Senju, Canon, Nidek, JINS, Kyowa Kirin); T. Yasukawa, Research Grant (Bayer, Santen, Chugai, Senju, Wakamoto), Consulting fees (Wakamoto), Honoraria for speakers (Novartis), Honoraria for speakers or their chairman (Bayer, Santen, Senju, Chugai), Participation on a Data Safety Monitoring Board or Advisory Board (Chugai); K. Yamashiro, Payment or honoraria for lectures (Novartis, Bayer, Santen, Senju, Chugai, Alcon, Kowa, Otsuka, HOYA, NIDEK, Asahi Kasei), Payment or honoraria for Consultant (Boehringer Ingelheim); S. Honda, Grants or contracts (Santen, Alcon, Senju, Chugai), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Chugai, Senju, Johnson & Johnson, Novartis); I. Maruko, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Novartis, Alcon, Santen, Senju, Topcon, Chugai, Canon, Nidek, Nikon), Patents planned, issued or pending (Jpn-patent (#7288276)); K. Kataoka, Grants (Chugai), Honoraria for lectures (Bayer, Chugai, Santen, Novartis, Senju, Boehringer Ingelheim, Canon, ZEISS), Participation on advisory Board (Chugai); Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid (Bayer).
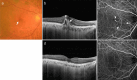
Figures






References
-
- Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–72. - PubMed
-
- Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22. - PubMed
-
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

