Small Molecule Catalyst for Peptide Synthesis
- PMID: 40658407
- PMCID: PMC12291467
- DOI: 10.1021/jacs.5c07242
Small Molecule Catalyst for Peptide Synthesis
Abstract
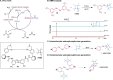
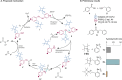
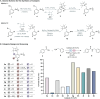
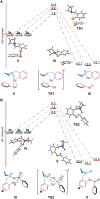
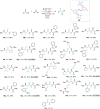
Peptide synthesis is a highly optimized process that has led to the production of new classes of therapeutics and materials. The process of peptide synthesis is straightforward: commercially available, orthogonally protected amino acids can be linked on the solid phase using highly efficient coupling agents. However, the simplicity of peptide synthesis masks a significant drawback of the current method: it is highly wasteful and utilizes a solvent that is facing restrictions on its use. A catalyst that allows solid phase synthesis of peptides in benign solvents without requirement for excess reagents and protected amino acids would have a significant impact. Here, we describe the development of a small molecule catalyst for peptide synthesis. The catalyst design incorporates redox recycling of diselenide and phosphine with air as the ultimate oxidant and phenylsilane as the ultimate reductant. The catalyst affords efficient coupling of amino acids in the solution and solid phase. Significantly, the catalyst functions with acetonitrile, bypassing the need for DMF. The current effort builds on mechanistic analysis of reaction rates and intermediates in our prior work which led to a hydrogen bonding catalyst: [Handoko; ; Panigrahi, N. R.; Arora, P. S. J. Am. Chem. Soc. 2022, 144, 3637-3643]. Here, we significantly simplified earlier designs to afford an easily accessible small molecule catalyst.
Figures


References
-
- Merrifield R. B.. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963;85:2149–2154. doi: 10.1021/ja00897a025. - DOI
-
- Carpino L. A., Han G. Y.. 9-Fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972;37:3404–3409. doi: 10.1021/jo00795a005. - DOI
-
- Albericio F., Bofill J. M., El-Faham A., Kates S. A.. Use of Onium Salt-Based Coupling Reagents in Peptide Synthesis1. J. Org. Chem. 1998;63:9678–9683. doi: 10.1021/jo980807y. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

