The locus coeruleus influences behavior by coordinating effective integration of fear memories and sensory input
- PMID: 40658743
- PMCID: PMC12273993
- DOI: 10.1371/journal.pbio.3003272
The locus coeruleus influences behavior by coordinating effective integration of fear memories and sensory input
Abstract
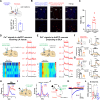
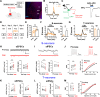
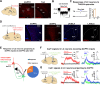
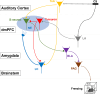
An essential function of memory is to guide behavior for better survival and adaptation. While memory formation has been extensively studied, far less is understood about how memory retrieval influences behaviors. In the auditory Pavlovian threat conditioning paradigm using C57BL/6J mice, retrieving a conditioned threat memory is associated with spiking in two dorsomedial prefrontal cortex (dmPFC) neurons with transient (T-neurons) and sustained (S-neurons) patterns. We show here that T-neurons and S-neurons are two distinct neuronal populations with different neuronal and synaptic properties and mRNA profiles. S-neuron spiking matches freezing behavior and is required for freezing. This sustained activity in S-neurons requires auditory inputs and the release of norepinephrine (NE) in the dmPFC. The activation of the locus coeruleus (LC) is initiated by dmPFC T-neuron inputs, sustained by auditory inputs, and is required for the transition to freezing by enhancing S-neuron activity. Interestingly, LC activation precipitates a brief period during which nonconditioned cues also induce freezing. Our findings highlight the critical contribution of the LC/NE system in the transition from memory to behavior, which coordinates the effective integration of memory, sensory inputs and emotional state for optimal adaptation.
Copyright: © 2025 Duan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

