The high burden of HEV infection in solid organ transplant recipients
- PMID: 40658785
- PMCID: PMC12263050
- DOI: 10.1097/HC9.0000000000000740
The high burden of HEV infection in solid organ transplant recipients
Abstract
Background: HEV is an important cause of morbidity in solid organ transplant (SOT) recipients. However, the total burden of hepatitis E, including subclinical infections in this group, is not well defined. We compared hepatitis E exposures in SOT recipients to non-transplant controls. We also examined the prevalence of rat HEV (rHEV), an emerging hepatitis agent, in this population.
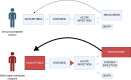
Methods: This study was conducted in the main SOT center in Hong Kong. Quantitative HEV IgG, RT-PCR, IgM, and IgG avidity assays were used to measure conventional HEV and rHEV exposures in 669 SOT recipients and 667 non-transplant hospitalized controls. Follow-up samples from a subset of SOT recipients were assessed to measure longitudinal HEV exposures.
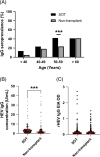
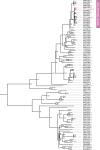
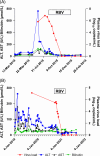
Results: Age-adjusted HEV IgG seroprevalence in SOT recipients (236/669; 35.3%) was significantly higher than non-transplant controls (185/667; 27.7%; p=0.001). Across baseline and follow-up samples, 25 (3.7%) SOT recipients had viremia (n=3) or serological evidence (n=22) of recent hepatitis E. The latter had IgM positivity (n=5), IgG seroconversion (n=16), or a 5-fold increase in longitudinal HEV IgG concentrations (n=1). Chronic hepatitis occurred in all 3 viremic individuals, while transient hepatitis was observed in 10/22 (45.4%) SOT recipients with serological evidence of recent hepatitis E. rHEV IgG levels were similar between SOT recipients and controls (p=0.424), but 2 viremic infections in the SOT group were due to rHEV and both turned chronic.
Conclusions: SOT recipients have higher hepatitis E seroprevalence than the non-transplant population. Increased exposure is driven by viremic infections and a significant burden of subclinical infections in Hong Kong. rHEV is an important cause of chronic hepatitis E in SOT recipients.
Keywords: follow-up studies; hepatitis E; organ transplantation; seroepidemiologic studies; transplant recipients.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Jianwen Situ and Siddharth Sridhar have filed a provisional patent application covering the utilization of hepatitis E virus-like particles described in this paper for serodiagnosis and vaccines. The remaining authors have no conflicts to report.
Figures

References
-
- Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. - PubMed
-
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–1271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

