The impact of BCLC recommendations on survival for patients with hepatocellular carcinoma
- PMID: 40658790
- PMCID: PMC12263035
- DOI: 10.1097/HC9.0000000000000750
The impact of BCLC recommendations on survival for patients with hepatocellular carcinoma
Abstract
Background: The Barcelona Clinic Liver Cancer (BCLC) system for HCC was updated in 2022. The aim of the study was to assess the suitability and impact on overall survival (OS) of BCLC_2022, along with "clinical decision-making" (CDM), using BCLC_2018 as a benchmark.
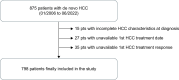
Methods: We retrospectively evaluated 798 patients with de novo HCC followed prospectively from 2006 to 2022: 187 in BCLC 0, 371 in A, 132 in B, 87 in C, and 21 in D, all managed by a multidisciplinary team. Patients were followed until death or at the end of the follow-up period in December 2022.
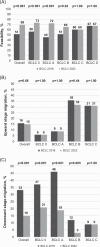
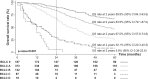
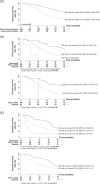
Results: The suitability of the algorithm increased from 51% for BCLC_2018 to 69% for BCLC_2022 (p<0.001). Among those treated with the newly introduced "lower priority options," 22% were in BCLC 0 and 37% in A, showing lower rates of complete response (CR) and shorter OS compared to first-line treatments. In BCLC 0 and A, CDM was associated with a significant decrease in "downward stage migration" with BCLC_2022 (from 33% to 16%, p<0.001). Conversely, in BCLC B and C, "upward stage migration" correlated with higher CR rates and longer OS [63 (36-72) vs. 28 (18-44) months, p=0.003 in BCLC B; 21 (15-44) vs. 11 (4-25) months, p<0.001 in BCLC C]. Independent predictors of mortality included AFP >200 ng/mL, Child-Pugh score C, advanced BCLC stage, and noncurative treatment.
Conclusions: BCLC_2022 and CDM provide greater flexibility in clinical practice without adversely affecting patient survival. Access to curative treatments improves the outcomes of selected patients in all stages.
Keywords: HCC; ablation; liver transplantation; surgery; systemic therapy.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Massimo Iavarone participated in the advisory board and received speaker fees from Gilead Sciences, Bayer, AstraZeneca, Roche, Roche Diagnostics, EISAI, IPSEN, and MSD. Barbara Antonelli received speaker fees and travel grants from Chiesi SpA and Roche. Eleonora Alimenti received speaker fees from Roche and Gilead. Giuseppe Cabibbo participated in advisory boards and received speaker fees from Bayer, Eisai, Ipsen, AstraZeneca, MSD, Roche, and Gilead. Pietro Lampertico participated in the advisory board and received speaker fees for AbbVie, Aligos, Altona, Antios, Eiger, Gilead Sciences, GlaxoSmithKline, Grifols, Janssen, MYR, Roboscreen, Roche Pharma/Diagnostics, and Vir. The remaining authors have no conflicts to report.
Figures

References
-
- Llovet J, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. - PubMed
-
- Forner A, Reig ME, Rodriguez de Lope C, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. - PubMed
-
- Sangiovanni A, Triolo M, Iavarone M, Forzenigo LV, Nicolini A, Rossi G, et al. Multimodality treatment of hepatocellular carcinoma: How field practice complies with international recommendations. Liver Int. 2018;38:1624–1634. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

