Communication initiated by hepatocytes: The driver of HSC activation and liver fibrosis
- PMID: 40658809
- PMCID: PMC12262956
- DOI: 10.1097/HC9.0000000000000753
Communication initiated by hepatocytes: The driver of HSC activation and liver fibrosis
Abstract
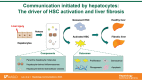
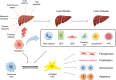
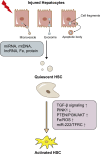
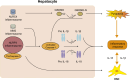
Liver fibrosis (LF) refers to the excessive deposition and abnormal distribution of the extracellular matrix (ECM) caused by acute or chronic liver injury, which affects the prognosis of liver diseases. Activated HSCs play a central role in LF through their ability to differentiate into myofibroblasts (MFBs) and secrete ECM. Intercellular communication within the liver is important for HSC activation and LF, whether in the initial or persistent stage. Hepatocytes (HCs), the most abundant cell type in the liver, are closely related to hepatic nutrition metabolism and detoxification. Moreover, HC damage is the initiating factor of LF, and interactions between HCs and HSCs may be the most critical event involved in the process of LF. This article reviews the intercellular communication between HCs and HSCs based on paracrine effects, extracellular vesicles, and inflammasomes, which is expected to lead to the development of effective antifibrotic strategies.
Keywords: HSC; extracellular vesicle; hepatocyte; inflammasome; intercellular communication; liver fibrosis; paracrine.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures
References
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
-
- Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–646. - PubMed
-
- Horn P, Tacke F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024;36:1439–1455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

