Prevention of extubation failure in neurocritical care patients with residual disorder of consciousness: the Brain-Injured Patients Extubation Readiness (BIPER) study protocol for a stepped-wedge cluster-randomised controlled trial
- PMID: 40659406
- PMCID: PMC12258314
- DOI: 10.1136/bmjopen-2025-104897
Prevention of extubation failure in neurocritical care patients with residual disorder of consciousness: the Brain-Injured Patients Extubation Readiness (BIPER) study protocol for a stepped-wedge cluster-randomised controlled trial
Abstract
Introduction: In the intensive care unit (ICU), brain-injured patients are frequently exposed to mechanical ventilation to protect the brain and preserve physiology. After intracranial pressure control and sedation withdrawal, this population is prone to residual disorder of consciousness and altered neurological control of respiratory drive, cough and airway protection. Consequently, extubation failure is more frequent than in general ICU patients, and there is no clear evidence-based clinical trigger for extubation. Different risk factors for extubation failure were described in observational trials, and clinical scores were constructed to detect patients at higher risk of extubation failure. Nevertheless, none of these scores were prospectively tested as interventional tools to prevent extubation failure. The Brain-Injured Patients Extubation Readiness (BIPER) study is an ongoing multicentre stepped-wedge cluster-randomised controlled trial aiming to test one of these scores as an intervention protocol to decrease extubation failure in neurocritical care patients with residual disorder of consciousness.
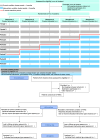
Methods and analysis: Trial design: Stepped-wedge cluster-randomised controlled trial with five groups of three to six clusters (20 ICUs). Groups of clusters are randomised to five possible sequences of nine periods with crossing from a control condition period (usual care for extubation) to an intervention condition period (BIPER-guided extubation protocol), separated by a 3-month transition period.
Participants: Participants are clinically stable brain-injured patients (18-75 years old), requiring more than 48 hours of invasive mechanical ventilation with residual disorder of consciousness after sedation withdrawal, and who achieved a spontaneous breathing trial.
Interventions: The control condition consists of extubation based on usual care and local practice. The intervention condition consists of extubation triggered by a clinical score evaluating deglutition, gag reflex, cough and visual tracking (Coma Recovery Scale-Revised Visual Scale).
Objective: To determine whether adoption of an extubation protocol based on a clinical score can lessen extubation failure compared with usual care in brain-injured patients with residual disorder of consciousness.
Outcome: The primary outcome measure is extubation failure, defined within 5 days following extubation. The key secondary outcome measure is time to effective extubation.
Randomisation: Clusters are allocated to sequence of treatments using random blocks randomisation. The constitution of groups of clusters was stratified according to planned recruitment of each centre.
Blinding: Investigators and outcome assessors are not blinded to condition allocation.Number of participants: 660 patients (220 in the control condition and 440 in the intervention condition).
Ethics and dissemination: The BIPER trial was approved by an independent ethics committee. The study began on 9 February 2020, and 571 participants are now included. Results will be published in an international peer-reviewed medical journal. TRIAL REGISTRATION NUMBER: NCT04080440.
Keywords: Brain Injuries; INTENSIVE & CRITICAL CARE; Neurological injury; Ventilators, Mechanical.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: RC reports receiving consulting fees from SOPHYSA and X.O outside the submitted work. ADJ declares fees for presentations for Medtronic, Drager Medical, Fisher & Paykel Healthcare, Viatris and Sanofi outside the submitted work. EF reports receiving consulting fees from Drager Medical and GE Healthcare, and honorariums for presentation from Fisher & Paykel Healthcare outside the submitted work. J-MC reports receiving consulting fees from Viatris, Amomed, Sedana Medical and Aspen, and honorariums for presentation from Shionogi, Gilead, Aspen, Drager Medical and Fisher & Paykel Healthcare outside the submitted work. MJ reports receiving lecture and consulting fees from AbbVie and Sedana Medical outside the submitted work. All other authors have no competing interest to declare.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
