The Helicobacter pylori AI-clinician harnesses artificial intelligence to personalise H. pylori treatment recommendations
- PMID: 40659612
- PMCID: PMC12259899
- DOI: 10.1038/s41467-025-61329-5
The Helicobacter pylori AI-clinician harnesses artificial intelligence to personalise H. pylori treatment recommendations
Abstract
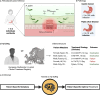
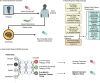
Helicobacter pylori (H. pylori) is the most common carcinogenic pathogen globally and the leading cause of gastric cancer. Here, we develop a reinforcement learning-based AI Clinician system to personalise treatment selection and evaluate its ability to improve eradication success compared to clinician-prescribed therapies. The model is trained and internally validated on 38,049 patients from the retrospective European Registry on Helicobacter pylori Management (Hp-EuReg), using independent state deep Q-learning (isDQN) to recommend optimal therapies based on patient characteristics such as age, sex, antibiotic allergies, country, and pre-treatment indication. In internal validation using real-world Hp-EuReg data, AI-recommended therapies achieve a 94.1% success rate (95% CI: 93.2-95.0%) versus 88.1% (95% CI: 87.7-88.4%) for clinician-prescribed therapies not aligned with AI suggestions-an improvement of 6.0%. Results are replicated in an external validation cohort (n = 7186), confirming generalisability. The AI system identifies optimal treatment strategies in key subgroups: 65% (n = 24,923) are recommended bismuth-based therapies, and 15% (n = 5898) non-bismuth quadruple therapies. Random forest modelling identifies region and concurrent medications as patient-specific drivers of AI recommendations. With nearly half the global population likely to contract H. pylori, this approach lays the foundation for future prospective clinical validation and shows the potential of AI to support clinical decision-making, enhance outcomes, and reduce gastric cancer burden.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Javier P. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly Spindler, Allergan, Diasorin, Richen, Biocodex and Juvisé. Olga P. Nyssen received research funding from Allergan, Mayoly Spindler, Richen, Biocodex and Juvisé. Drs Kirill Veselkov, Ivan Laponogov, and Dennis Veselkov are affiliated with Intelligify Ltd, an AI consultancy company, which was not involved in the research, analysis, or interpretation of the results presented in this study. Tania Fleitas Kanonnikoff discloses advisory roles honoraria from Amgen, AstraZeneca, Beigene, BMS and MSD. Institutional research funding from Gilead. Speaker honoraria from Amgen, Servier, BMS, MSD, Lilly, Roche, Bayer. The remaining authors declare no conflicts of interest. POLICY DISCLOSURE-USE OF CLINICAL DATA. This study involves the secondary analysis of de-identified clinical data obtained from the European Registry on Helicobacter pylori Management (Hp-EuReg). The data were originally collected by the Hp-EuReg consortium across multiple centres in Europe under appropriate ethical approvals and patient consent at the time of collection. No new data were collected for the purposes of this analysis, and the authors were not involved in direct recruitment or interaction with study participants. All analyses were conducted on anonymised data in accordance with applicable data protection and ethical guidelines.
Figures


References
-
- Suerbaum, S. & Michetti, P. Helicobacter pylori infection. N. Engl. J. Med.347, 1175–1186 (2002). - PubMed
-
- Sipponen, P. et al. Cumulative 10-year risk of symptomatic duodenal and gastric ulcer in patients with or without chronic gastritis: a clinical follow-up study of 454 outpatients. Scand. J. Gastroenterol.25, 966–973 (1990). - PubMed
-
- Liou, J.-M. et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut69, 2093–2112 (2020). - PubMed
-
- de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health8, e180–e190 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

