Hippo pathway and NLRP3-driven NETosis in macrophages: Mechanisms of viral pneumoniaaggravation
- PMID: 40659620
- PMCID: PMC12260020
- DOI: 10.1038/s41420-025-02556-z
Hippo pathway and NLRP3-driven NETosis in macrophages: Mechanisms of viral pneumoniaaggravation
Abstract
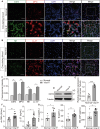
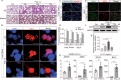
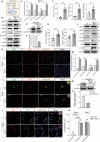
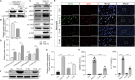
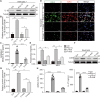
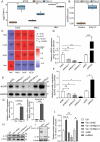
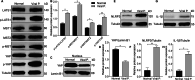
Severe viral infections can precipitate acute lung injury, resulting in significant morbidity and mortality. While NETosis serves as an important defense mechanism against pathogens and viruses, its excessive or dysregulated activation may contribute to pulmonary damage. In this study, elevated levels of NETosis were detected in the peripheral blood of patients with viral pneumonia. To further investigate the relationship between NETosis and virus-induced acute lung injury, a murine model was established using intratracheal administration of poly(I:C), a synthetic analog of double-stranded RNA that mimics viral infection. NETosis biomarkers were assessed in both patients and poly(I:C)-stimulated mice. In addition, we examined the role of the Hippo signaling pathway and its downstream mediators, including inflammatory factors and chemokines. Enhanced NETosis and activation of the Hippo pathway were observed in the lungs of poly(I:C)-treated mice, along with elevated levels of IL-1β in isolated macrophages. These effects were mitigated by Hippo pathway inhibitors. Co-culture experiments confirmed that IL-1β promotes NETosis, while NLRP3, acting downstream of the Hippo pathway, was responsible for IL-1β secretion. Patients with viral pneumonia showed increased NLRP3 and IL-1β expression in monocyte-derived macrophages compared to healthy controls. Overall, our findings indicate that activation of the Hippo pathway in macrophages during poly(I:C) exposure upregulates NLRP3 and IL-1β expression, thereby promoting NETosis and exacerbating virus-induced lung injury. This study highlights a potential therapeutic target to reduce lung damage caused by viral infections.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Gu S, Pan P, Wang J, Shi Y, Shi F, Zhang Y, et al. Exploring influenza a virus-induced lung injury and immune response based on humanized lung-on-chip. Discov Med. 2023;35:539–52. - PubMed
LinkOut - more resources
Full Text Sources

