Early identification of individuals at risk for multiple sclerosis by quantification of EBNA-1381-452-specific antibody titers
- PMID: 40659624
- PMCID: PMC12259891
- DOI: 10.1038/s41467-025-61751-9
Early identification of individuals at risk for multiple sclerosis by quantification of EBNA-1381-452-specific antibody titers
Abstract
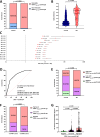
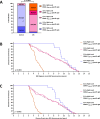
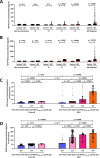
Multiple sclerosis (MS) is an immune-mediated demyelinating disease. Epstein-Barr virus (EBV) encodes for the EBNA-1381-452 region that induces autoreactive antibody responses, which are likely critically involved in MS pathogenesis. Here we investigate whether these EBNA-1381-452-specific antibodies can serve as a biomarker to identify at-risk individuals for MS. We quantify EBNA-1381-452-specific antibody titers from 324 relapsing-remitting MS patients and 324 matched controls in longitudinal follow-up plasma samples, starting from the individual's EBV-seroconversion. In MS patients, significantly elevated EBNA-1381-452-specific IgG titers are identified that are increased already as early as nine months after EBV-seroconversion (OR:5.7; 95% CI: 4.1-8.1; P < 0.0001) and a median 5.4 years prior to MS diagnosis. Especially, the presence of continuously high EBNA-1381-452-specific antibody titers is associated with a more rapid MS diagnosis after EBV-seroconversion (P < 0.0001). Thus, the quantification of EBNA-1381-452-specific IgG antibody levels may provide a prognostic biomarker to determine the individual's risk for the diagnosis of MS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Sci. (N. Y., NY)375, 296–301 (2022). - PubMed
-
- Ascherio, A. et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. Jama286, 3083–3088 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

