CD8+GZMK+CD27+CCR7+ T cells mobilized by splenic sympathetic nerves aggravate brain ischemia‒reperfusion injury via CCL19-positive endothelial cells
- PMID: 40659887
- PMCID: PMC12398583
- DOI: 10.1038/s41423-025-01311-9
CD8+GZMK+CD27+CCR7+ T cells mobilized by splenic sympathetic nerves aggravate brain ischemia‒reperfusion injury via CCL19-positive endothelial cells
Abstract
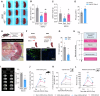
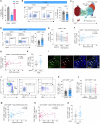
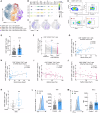
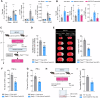
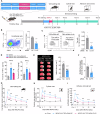
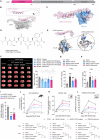
Splenic sympathetic activity critically modulates peripheral immunity after ischemic stroke, thus intervention in spleen sympathetic activity represents a promising therapeutic strategy for stroke. However, the mechanisms underlying spleen-brain-immune axis communication remain poorly understood. Here, we utilized a surgical denervation protocol to perform splenic sympathetic denervation (SDN), which significantly attenuated brain injury following stroke. Through single-cell RNA sequencing, we identified a novel GZMK+CD8+CD27+CCR7+ T-cell subset in patients with acute ischemic stroke (AIS), which we designated stroke-associated T (Tsa) cells. The expansion of Tsa cells was positively correlated with the severity of clinical symptoms and was driven by the splenic sympathetic nervous system. Stroke-induced sympathetic activation triggers the release of splenic norepinephrine (NE), which preferentially signals through ADRB2 on Tsa cells to promote their mobilization. Additionally, ischemic injury induces endothelial cell-specific expression of CCL19, which chemoattracts Tsa cells into the brain parenchyma via their cognate CCR7 receptor, exacerbating neuroinflammatory injury and neurological deficits in a transient middle cerebral artery occlusion (tMCAO) mouse model. We developed a CCR7-targeting peptide to disrupt this chemotactic axis and reduce T-cell infiltration, thereby mitigating brain injury. Our findings highlight SDN as a promising therapeutic strategy to attenuate ischemia‒reperfusion injury and suggest its potential as an adjunctive therapy for reperfusion treatment in AIS patients.
Keywords: Acute ischemic stroke; CCL19; CCR7; Functional recovery; Splenic sympathetic denervation; Stroke-associated T cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

