Effectiveness of single-pill combination olmesartan/amlodipine/hydrochlorothiazide therapy in patients with apparent resistant hypertension
- PMID: 40659924
- PMCID: PMC12417203
- DOI: 10.1038/s41371-025-01043-3
Effectiveness of single-pill combination olmesartan/amlodipine/hydrochlorothiazide therapy in patients with apparent resistant hypertension
Abstract
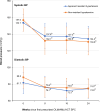
This pioneering study investigated the real-life effectiveness of single-pill combination (SPC) antihypertensive therapy in patients with uncontrolled blood pressure (BP) and cardiovascular risk factors. After treatment with olmesartan medoxomil/amlodipine besylate/hydrochlorothiazide for 24 weeks, adults with apparent resistant hypertension had significantly reduced systolic BP and approximately 80% achieved target BP. SPC therapy may improve adherence in patients with pseudo-resistant hypertension and is a potentially effective treatment for resistant hypertension.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Jaewon Oh, Wonho Kim, Gee-Hee Kim, Hack-Lyoung, Sang-Don Park, Kyung Wan Min, Dongkeun Hyun, Jun Hwa Hong, and Soo Lim declare that they have no competing interests. Ji-Hwan Bae is a full-time employee of Daiichi Sankyo Korea Co., Ltd. Jinho Shin received honoraria from Daiichi Sankyo, Organon, Bristol Myers Squibb, Menarini, Sanofi, Hanmi, Boryung, Chong Kun Dang, and Daewoong, and grants from Sanofi and Hanmi. Ethical approval: This study was conducted according to ethical principles established by the Declaration of Helsinki. The Review Boards of participating institutions approved the protocol. All patients provided informed written consent.
Figures
References
-
- Schiffrin EL, Fisher NDL. Diagnosis and management of resistant hypertension. BMJ. 2024;385:e079108. - PubMed
-
- Brant LCC, Passaglia LG, Pinto-Filho MM, de Castilho FM, Ribeiro ALP, Nascimento BR. The burden of resistant hypertension across the world. Curr Hypertens Rep. 2022;24:55–66. - PubMed
-
- Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

