Comparing eptinezumab with onabotulinumtoxinA in the treatment of chronic migraine: a real-world evidence study
- PMID: 40660133
- PMCID: PMC12261790
- DOI: 10.1186/s10194-025-02106-z
Comparing eptinezumab with onabotulinumtoxinA in the treatment of chronic migraine: a real-world evidence study
Abstract
Background: Migraine, a spectrum of episodic headache disorders common in the global population, may evolve into a very debilitating chronic condition, usually underdiagnosed, poorly recognized and difficult to manage. Neurotransmission in the trigeminovascular sensory nervous system, particularly involving calcitonin gene-related peptide (CGRP), has emerged as a prime therapeutic target for the treatment of chronic migraine. Here we investigated differences in efficacy of eptinezumab (a monoclonal antibody [mAb] targetting CGRP) and onabotulinumtoxinA (an inhibitor of neurotransmitter release) in an Italian real-world setting in which medical medication overuse headache (MOH) is prevalent.
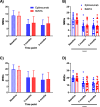
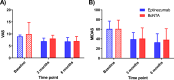
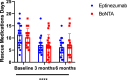
Methods: Forty chronic migraine patients refractory to standard treatments were observed, of which 15 were over 50 years old, and 4 exceeded 65 years. The primary endpoint of efficacy was reduction of monthly migraine days (MMDs) collected at two time points: after 3 and 6 months, respectively. The secondary endpoints, also performed after 3 and 6 months, include reduction of monthly headache days (MHDs), days in need of rescue medications, diminished pain intensity and on disability measured as Migraine Disability Assessment (MIDAS). Statistical differences were analysed using two-way analysis of variance with measurement repetition tests (Two-way RM ANOVA, followed by Bonferroni or Sidak test).
Results: Both eptinezumab and onabotulinumtoxinA demonstrated efficacy at 3 and 6 months, with no statistically significant differences between them for either primary or secondary endpoints. Both treatments had a more pronounced effect on MMDs and MHDs than on the reduction of intensity and disability, and reduced the need for rescue medications at 3 and 6 months. After 3 months, onabotulinumtoxinA displays a trend to reduce the need to rescue medication in a higher number of patients compared to eptinezumab.
Conclusions: OnabotulinumtoxinA was as effective as eptinezumab for chronic migraine prophylaxis according to both primary and secondary endpoints and, therefore, is useful as an alternative or adjunct therapy to mAbs or gepants.
Keywords: Chronic migraine; Efficacy; Eptinezumab; OnabotulinutoxinA; Pharmacovigilance; Real-world; Rescue medications; Safety.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by Calabria Region Ethics Committee with number 303/2024 and conducted in accordance with the Declaration of Helsinki. Competing interests: The authors declare that they have no competing interests. GWL has received funding from Abbvie Inc. for basic non-clinical research unrelated to the present study. Otherwise, the authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

