Royal jelly alleviates gemcitabine-induced ovarian toxicity: an investigation on rat models
- PMID: 40660206
- PMCID: PMC12261596
- DOI: 10.1186/s12906-025-05013-7
Royal jelly alleviates gemcitabine-induced ovarian toxicity: an investigation on rat models
Abstract
Background: Royal Jelly (RJ), an important product of apitherapy, has been traditionally used for its various health benefits, particularly for its anti-inflammatory, antioxidant, and immune-modulatory properties. RJ's ability to reduce oxidative stress, inflammation, and cellular damage has made it a promising candidate for preserving ovarian function and fertility during cancer treatments. Gemcitabine (GEM) is an antimetabolite chemotherapeutic drug known to cause ovarian toxicity. To date, there has been no study evaluating the protective effects of RJ specifically against GEM-induced ovarian damage, making this an original contribution to the field. This study investigated RJ's protective effects against GEM-induced ovarian toxicity in rats.
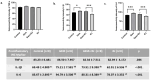
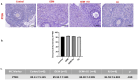
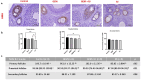
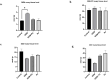
Method: Thirty-two female Wistar-Albino rats were divided into four groups: Control, RJ, GEM, and GEM + RJ. GEM (200 mg/kg) was administered intraperitoneally, while RJ (100 mg/kg) was given orally for one week before GEM administration. Histopathological, immunohistochemical, and biochemical analyses were performed to assess ovarian tissue damage, inflammation, and oxidative stress markers.
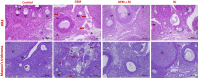
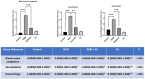
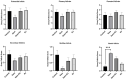
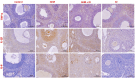
Results: GEM treatment caused ovarian damage, including vascular congestion, vacuolization, and Hemorrhage. RJ partially suppressed GEM-induced increases in TNF-α, IL-1β, and IL-6 levels and enhanced AMH expression only in primary follicles. Additionally, RJ only partially lowered FSH levels while increasing LH levels. RJ also counteracted GEM-induced oxidative stress by reducing MDA levels and partially enhancing SOD and CAT activity. GEM caused ovarian damage, including vascular congestion, vacuolization, hemorrhage, inflammation, and oxidative stress.
Conclusions: RJ partially reduced inflammatory response and oxidative stress, supported follicular development, increased AMH expression, and alleviated histopathological damage. This original study highlights the potential of RJ as a natural adjunct to preserve ovarian reserve during gemcitabine chemotherapy, while emphasizing the need for further research to determine its long-term effects and optimal dosage.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Animal Experiments Local Ethics Committee of the Erciyes University Animal Experiments Local Ethics Committee (Ethics Committee No 2022-22/189). No human participants were involved in this study, and therefore, consent to participate is not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Effect of autologous cytokine-rich serum and platelet-rich plasma administration on oxidative status, minerals and proinflammatory cytokines in brain and serum in cyclophosphamide-induced ovarian failure.J Mol Histol. 2025 May 19;56(3):159. doi: 10.1007/s10735-025-10448-w. J Mol Histol. 2025. PMID: 40387948
-
Protective effects of Tribulus Terrestris extract on cisplatin-induced ovarian damage: Antioxidants and anti-inflammatory insights.Hum Exp Toxicol. 2025 Jan-Dec;44:9603271251353492. doi: 10.1177/09603271251353492. Epub 2025 Jul 18. Hum Exp Toxicol. 2025. PMID: 40676950
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Royal jelly and doxorubicin suppressed tumor cells in the xenograft model of lung cancer via the STAT3/FOXM1/ATG7 signaling pathways in athymic nude mice: a biochemical, immunohistochemically and molecular approach.Toxicol Res (Camb). 2025 Mar 27;14(2):tfaf042. doi: 10.1093/toxres/tfaf042. eCollection 2025 Apr. Toxicol Res (Camb). 2025. PMID: 40161259
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9(4):47–47. - PubMed
-
- Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB. Evaluation of the antitumor activity of gemcitabine (2′, 2′-difluoro-2′-deoxycytidine). Cancer Res. 1990;50(14):4417–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

