Hyaluronic acid-tailored prodrug nanoplatforms for efficiently overcoming colorectal cancer chemoresistance and recurrence by synergistic inhibition of cancer cell stemness
- PMID: 40660238
- PMCID: PMC12261673
- DOI: 10.1186/s12951-025-03484-x
Hyaluronic acid-tailored prodrug nanoplatforms for efficiently overcoming colorectal cancer chemoresistance and recurrence by synergistic inhibition of cancer cell stemness
Abstract
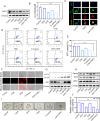
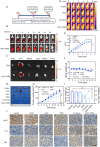
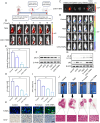
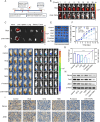
A subset of residual colorectal cancer (CRC) cells with stemness features exhibits a transient adaptive resistance after chemotherapy, limiting durable therapeutic benefits and even accelerating tumor recurrence. To tackle this problem, we have developed a targeted polymer prodrug nanoplatform (CHH-T/NPs) capable of synergistically inhibiting cancer cell stemness by modulating intracellular metabolism and inhibiting protective autophagy. Hyaluronic acid (HA) acts as a tumor-targeting molecular backbone, α-cyanohydroxycinnamic acid (CHC) is an inhibitor of monocarboxylic acid transporter 1 (MCT1), and hydroxychloroquine sulfate (HCQ) is an inhibitor of autophagy. These compounds were loaded on the HA backbone to form a polymeric prodrug, CHH, with pH-responsive ester bonds. CHH was self-assembled with mitochondria-targeting IR820 (T820), resulting in the formation of CHH-T/NPs. CHC and T820 disrupted cellular metabolism by inducing mitochondrial dysfunction and inhibiting lactate transport, leading to a synergistic inhibition of cancer cell stemness. Simultaneously, HCQ effectively inhibited autophagy to disrupt the self-protection mechanism of CRC cells. As anticipated, CHH-T/NPs effectively suppressed the chemoresistance and postoperative recurrence of CRC in subcutaneous and in situ tumors models. Taken together, this approach presents a promising strategy for overcoming CRC chemoresistance and recurrence through the synergistic inhibition of cancer cell stemness.
Keywords: Chemoresistance; Colorectal cancer; Recurrence; Site-specific polymer prodrug; Stem maintenance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal protocols were approved by the Administration Committee of Experimental Animals in Sichuan Province and the Ethics Committee of Sichuan Provincial People’s Hospital (SYXK, Sichuan, 2018-058). Consent for publication: All authors of this study agreed to publish. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Forkhead-box A3 (FOXA3) Represses Cancer Stemness and Partially Potentiates Chemosensitivity by Targeting Metastasis-Associated in Colon Cancer 1 (MACC1) Signaling Pathway in Colorectal Cancer Cells.Curr Cancer Drug Targets. 2021;21(3):254-267. doi: 10.2174/1568009620666201207150632. Curr Cancer Drug Targets. 2021. PMID: 33292133
-
Curcumin-based polymer prodrug nanoplatform for high-efficiency immunotherapy by synergistically suppression of head and neck cancer cell stemness.J Nanobiotechnology. 2025 Jul 11;23(1):500. doi: 10.1186/s12951-025-03559-9. J Nanobiotechnology. 2025. PMID: 40646575 Free PMC article.
-
EZH2 promotes chemoresistance in colorectal cancer by inhibiting autophagy through NRP1 suppression.Biochem J. 2025 May 21;482(10):569-581. doi: 10.1042/BCJ20240607. Biochem J. 2025. PMID: 40314246 Free PMC article.
-
Hyaluronic acid-based drug nanocarriers as a novel drug delivery system for cancer chemotherapy: A systematic review.Daru. 2021 Dec;29(2):439-447. doi: 10.1007/s40199-021-00416-6. Epub 2021 Sep 9. Daru. 2021. PMID: 34499323 Free PMC article.
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
References
-
- van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer, nature reviews. Clin Oncol. 2017;14(5):297–315. - PubMed
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer Stat 2023 CA: cancer J Clin. 2023;73(3):233–54. - PubMed
-
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London England). 2019;394(10207):1467–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

