Co-morbid mechanisms of intervertebral disc degeneration and osteoporosis: biomechanical coupling and molecular pathways synergistically driving degenerative lesions
- PMID: 40660249
- PMCID: PMC12261636
- DOI: 10.1186/s13018-025-06075-6
Co-morbid mechanisms of intervertebral disc degeneration and osteoporosis: biomechanical coupling and molecular pathways synergistically driving degenerative lesions
Abstract
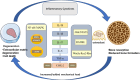
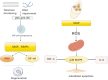
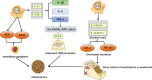
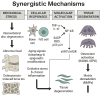
Degenerative orthopedic illnesses, such as osteoporosis (OP) and intervertebral disc degeneration (IVDD), are common in the elderly and are defined by loss of bone mass and degradation of the intervertebral disc matrix, respectively. These conditions cause persistent pain and disability. Although more research has been done on the two diseases' distinct causes, epidemiology indicates that their co-morbidity incidence has dramatically grown, pointing to a synergistic pathogenic network. Big data-driven dual-disease research offers a fresh approach to exposing the pathophysiology of co-morbidities, as the conventional single-disease research model makes it challenging to examine the interaction mechanisms. Research has indicated that the co-morbidities are primarily caused by metabolic and biomechanical disorders: A vicious cycle of "mechanics-bone loss" is created when deteriorated discs hasten the breakdown of the bone microarchitecture through spinal instability, while OP-induced reductions in bone mineral density (BMD) cause aberrant loading of the intervertebral discs. At the molecular level, inflammatory factors like TNF-α and IL-1β contribute to the "inflammation-degeneration-bone loss" axis by activating the NF-κB pathway, which in turn promotes osteoclast activation (RANKL/OPG imbalance) and intervertebral disc matrix breakdown (upregulation of matrix metalloproteinases (MMPs)). Convergence of senescence signaling makes the co-morbid process worse: SASP/ROS-induced apoptosis in bone tissue and p53/p21-mediated senescence in disc nucleus pulposus cells work together to alter microenvironmental homeostasis through SASP secretion simultaneously. Autophagy and epigenetic modification are both modulated via the SIRT1/mTOR pathway, while autophagy and epigenetic modification are regulated by exosomal miRNAs (e.g., miR-31, miR-143-5p). 143-5p) mediate the signaling of trans-tissue senescence. Oxidative stress and chronic inflammation are amplified by immunometabolic reprogramming (macrophage M1 polarization, NLRP3 inflammatory vesicle activation) and anomalies in mitochondrial energy metabolism (reduced ATP generation). To study the mechanisms of bone-disc mechanotransduction and molecular dialogue, create multi-targeted synergistic intervention strategies, and screen bi-directional regulatory biomarkers, we must combine biomechanical modeling and single-cell multi-omics technology in the future. This will provide theoretical advances for the development of an accurate therapeutic system that considers tissue homeostasis.
Keywords: Aging; Biomechanical imbalance; Disc degeneration; Macrophage polarization; Osteoporosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529–55. - PubMed
-
- Pei S, et al. RhTSG-6 inhibits IL-1β-induced extracellular matrix degradation and apoptosis by suppressing the p38, and JNK pathways in nucleus pulposus cells. Folia histochemica et cytobiologica; 2020. - PubMed
-
- Wang M, et al. Electroacupuncture regulates SIRT1/p53/p21 signaling pathway to prevent stress-induced premature senescence of nucleus pulposus cells in degenerative intervertebral discs. Int Immunopharmacol. 2025;148:114114. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

